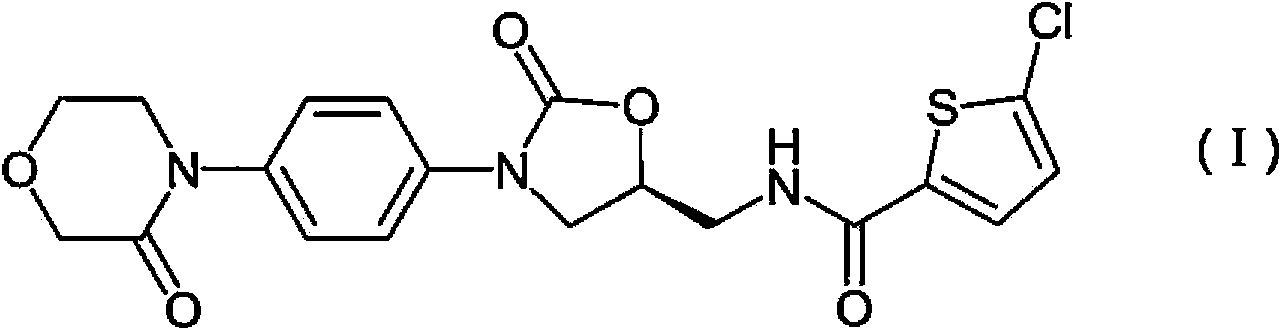
Rivaroxaban purification method
A technology of rivaroxaban and crude products, applied in the direction of organic chemistry and the like, can solve the problems of easy corrosion reaction equipment, high toxicity of acetonitrile, unsuitable for industrialization, unsuitable for industrialized production, etc., and achieves the effect of facilitating industrialized production
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] Embodiment 1: Preparation of crude product of rivaroxaban
[0029] Preparation method of reference (J.Med.Chem.48:5900-5908, 2005).
[0030] The first step: 2-((2R)-2-hydroxyl-3-{[4-(3-oxomorpholin-4-yl)phenyl]amino}propyl)-1H-isoindole-1, Preparation of 3(2H)-diketone (IV)
[0031] 2-[(2S)-Oxiran-2-ylmethyl]-1H-isoindole-1,3(2H)-dione (II) (5.68g, 27.9mmol) and 4-(4 -Aminophenyl)-3-morpholinone (III) (5.37g, 27.9mmol) was suspended in ethanol-water (9:1, 140ml) solution, and refluxed for 14h (the raw materials gradually dissolved and formed after a period of time) precipitate), the precipitate was filtered off, washed with ether and dried under vacuum, the combined mother liquors were concentrated under reduced pressure and a second portion of 2-[(2S)-oxiran-2-ylmethyl]-1H-isoindrate was added Indole-1,3(2H)-diketone (II) (2.84g, 14.0mmol) in ethanol-water (9:1, 70ml) suspension, reflux reaction for 13h, filtered, washed with ether, dried under vacuum, 10.14 g of w...
Embodiment 2
[0038] Embodiment 2: the refinement of rivaroxaban crude product
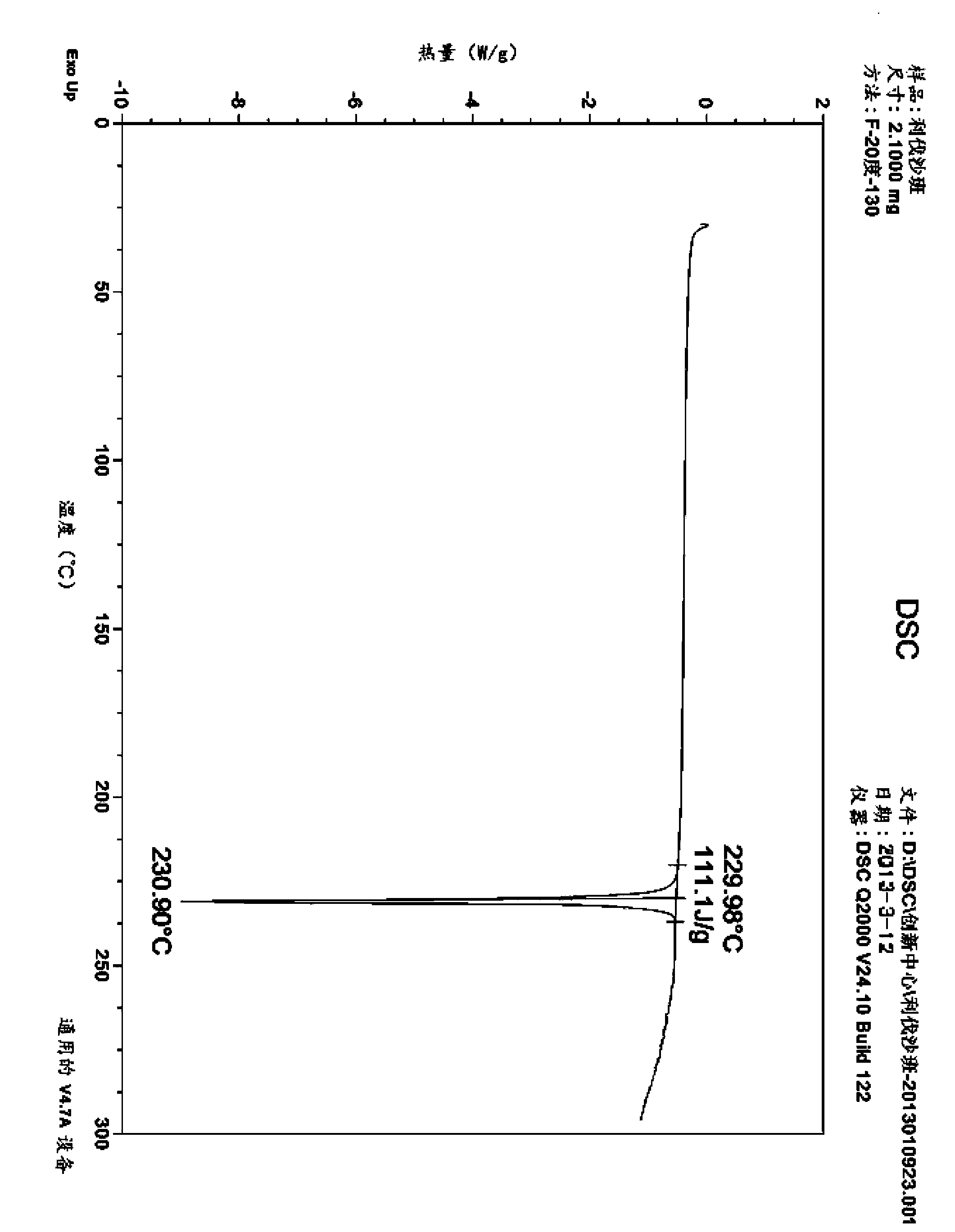
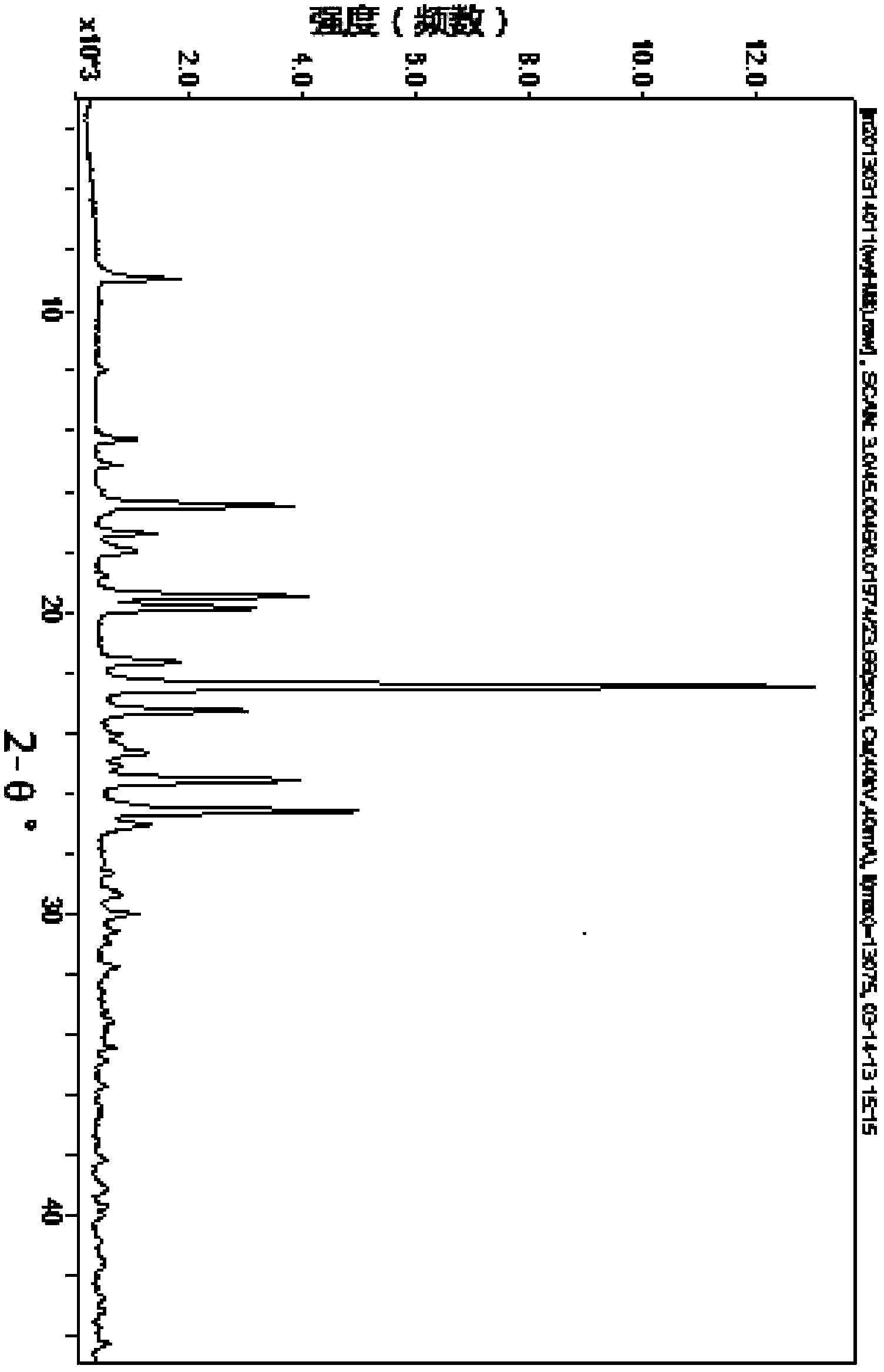
[0039] Suspend the crude product of 4 g of rivaroxaban in 60 ml of ethylene glycol methyl ether and heat it to 125 ° C, add 0.4 g of activated carbon, stir the resulting solution at this temperature for 10 minutes, then filter while it is hot, and cool the mother liquor to room temperature. Suction filtration, the precipitated product was filtered out, washed with ethylene glycol methyl ether, and dried to obtain 3.46 g of white solid, recrystallization yield: 86.5%. HPLC purity 99.74%, ee%: 99.96%. DSC melting temperature: 230°C. 1 HNMR (400MHz, d 6 -DMSO): 9.00-8.93(t, J=5.6Hz, 6Hz, 1H), 7.70(d, J=4Hz, 1H), 7.60-7.53(d, J=8.8Hz, 2H), 7.44-7.37(d , J=8.2Hz, 2H), 7.21-7.17(d, J=4Hz, 1H), 4.80-4.89(m, 1H), 4.24-4.15(m, 3H), 4.00-3.94(m, 2H), 3.89 -3.82(m, 1H), 3.75-3.68(m, 2H), 3.64-3.58(t, J=5.6Hz, 2H); the 2θ angles of powder diffraction are: 8.960°, 16.481°, 25.582°, 26.608°, 19.483°, 19.877°, 21.654°, ...
Embodiment 3
[0040] Embodiment 3: the refinement of rivaroxaban crude product
[0041] Suspend the crude product of 5 g of rivaroxaban in 50 ml of ethylene glycol methyl ether and heat it to 140 ° C, add 1.5 g of activated carbon, stir the resulting solution at this temperature for 10 minutes, then filter while it is hot, and cool the mother liquor to 60°C. Suction filtration, the precipitated product was filtered out, washed with ethylene glycol methyl ether, and dried to obtain 4.3 g of white solid; recrystallization yield: 86.0%. HPLC purity 99.67%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com