Exenatide <18>F marker, and preparation method and application thereof
A marker, 18F technology, applied in the preparation method of peptides, chemical instruments and methods, peptides, etc., can solve the problems of not being able to know the labeling site and affect the diagnosis, and achieve high labeling efficiency, clear image contrast, and broad prospects Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
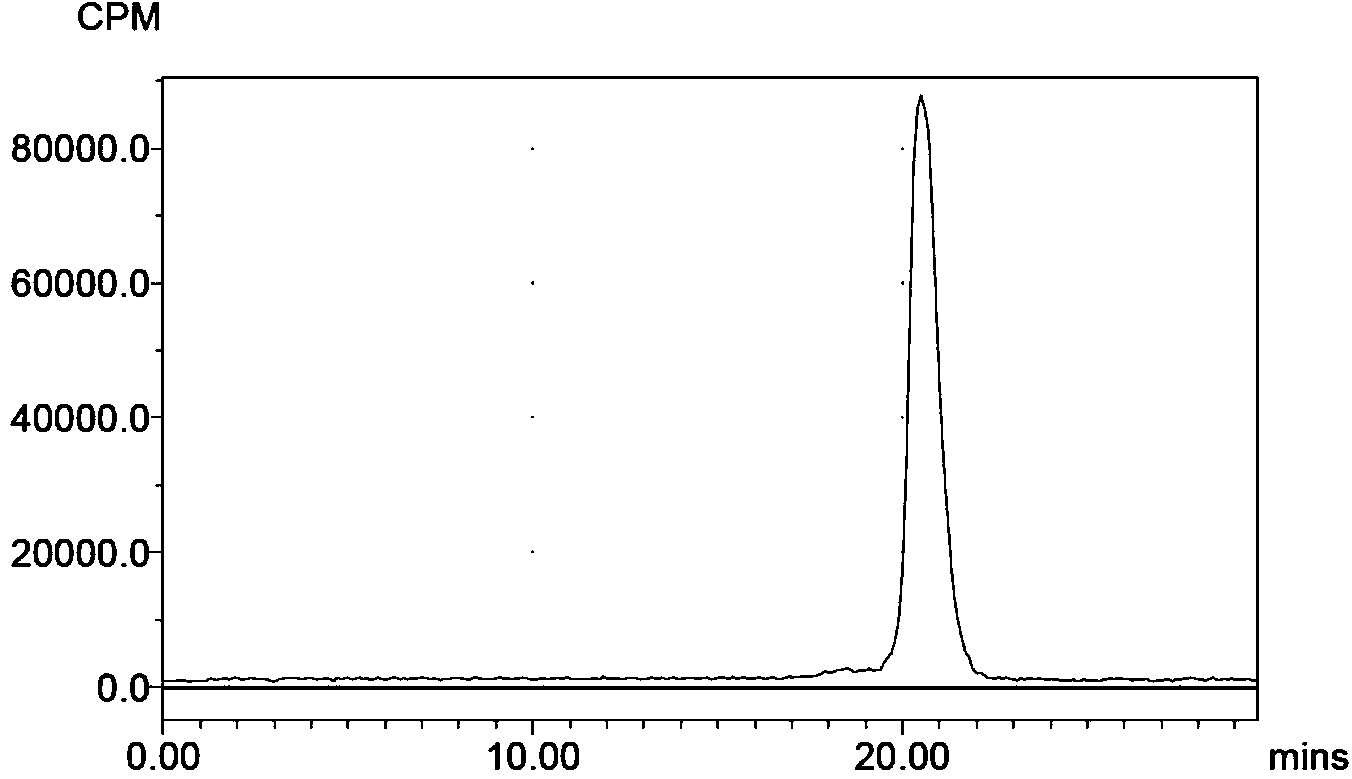
[0049] Described in this example 18 F-FBEM-Cys 39 -The preparation of Exenatide marker comprises the following steps:
[0050] (1) Prepared by cyclotron 18 f - , and added to 0.1ml containing K 2 CO 3 (3mg) and Kryptofix 222 (15 mg) in a reaction flask of acetonitrile-water solution, by azeotropic evaporation to obtain anhydrous 18 f - ; take anhydrous 18 f - Add 4-trimethylamine ethyl benzoate trifluorosulfonate (5 mg, dissolved in 0.3 ml anhydrous dimethyl sulfoxide (DMSO)), heat to 115 ° C for 20 min, after cooling the reaction solution, then add 0.1 M NaOH for hydrolysis After acidification with 1M HCl; the reaction solution was purified by an activated Sep-Pak C18 column, and the product was rinsed with dichloromethane and dried with nitrogen to obtain 4- 18 F-benzoic acid;
[0051] (2) Take the prepared 4- 18 F-benzoic acid, add 0.3ml of DMSO solution containing 15mg2-aminoethylmaleimide, 10mg N, N-diisopropylethylamine (DIEA) and 20μL diethyl cyanophosphate,...
experiment example
[0059] 1. Small animal PET imaging of nude mouse model
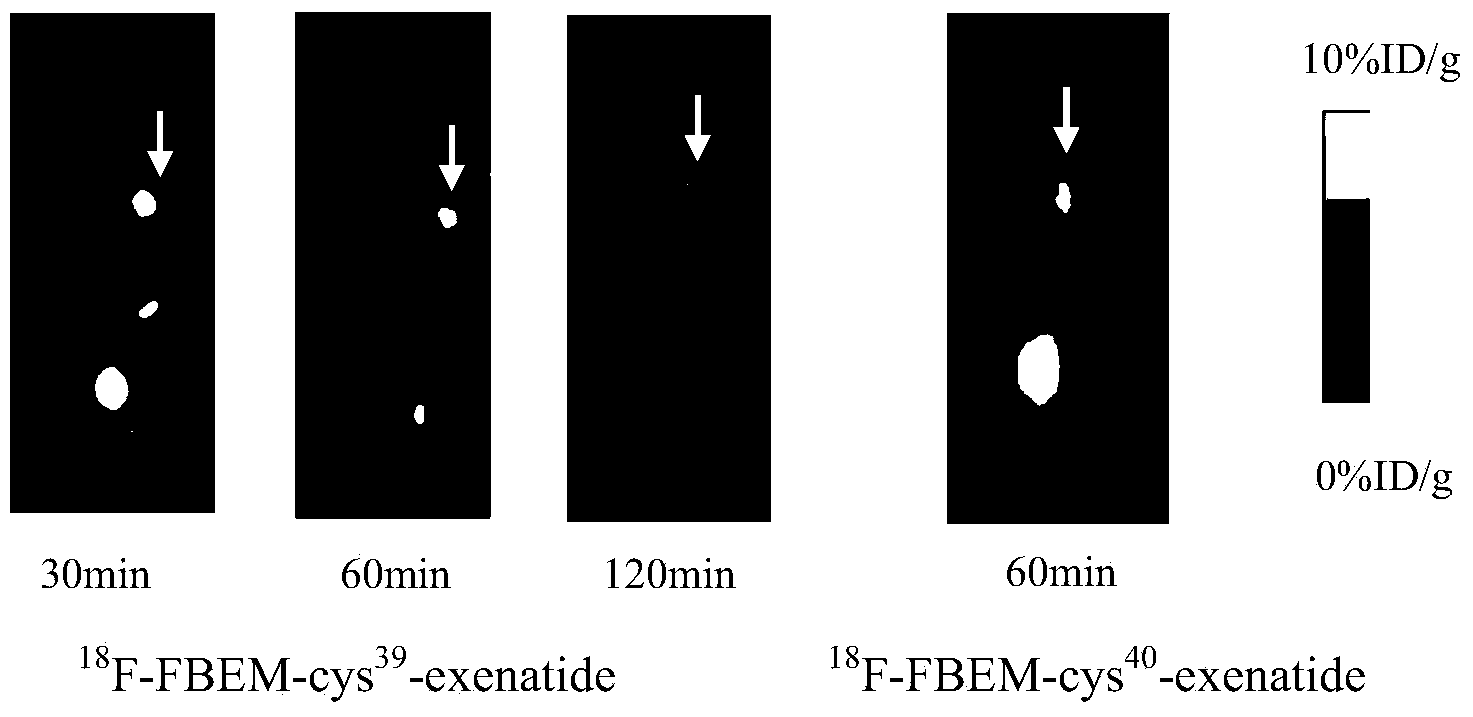
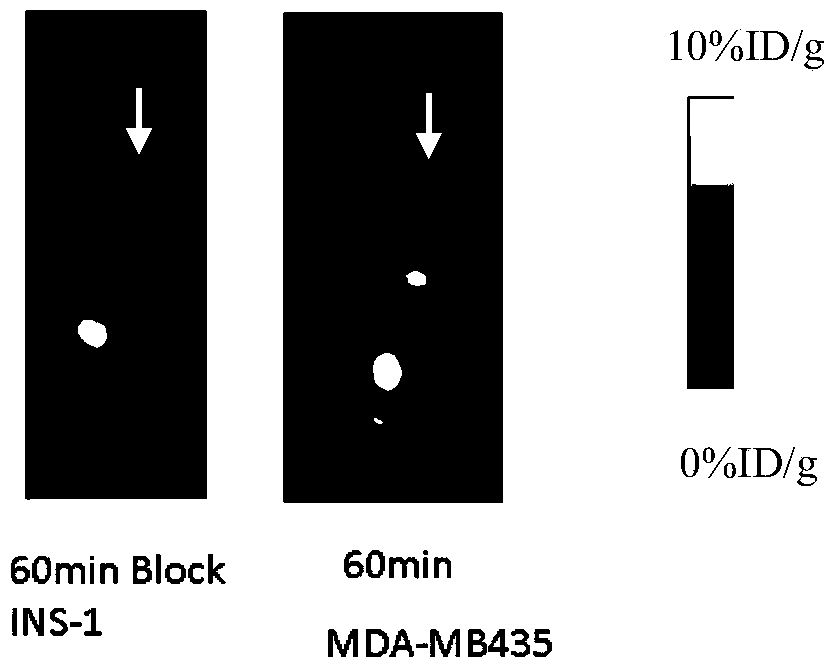
[0060] Insulinoma INS-1 nude mice bearing GLP-1 receptor positive expression were placed on small animal PET beds, anesthetized with isoflurane, and fixed with adhesive tape. Model mice were injected with the labeled product obtained in Example 1 through the tail vein, 18 F-FBEM-Cys 39 - Exenatide in saline solution (1.85 MBq, 0.2 mL). 30 minutes, 1 hour, and 2 hours after injection, microPET imaging was performed, and the results were as follows: Figure 2A As shown, the arrow in the figure indicates the tumor.
[0061] As a comparison, another INS-1 nude mouse with insulinoma positive for GLP-1 receptor expression was placed on a small animal PET bed, anesthetized with isoflurane, and fixed with adhesive tape. Model mice were injected with the labeled product obtained in Comparative Example 1 through the tail vein, 18 F-FBEM-Cys 40 - Exenatide in saline solution (1.85 MBq, 0.2 mL). MicroPET imaging was performed...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com