Isotopic abundance detection method for D, 13C or 15N labeled organic compounds
A technology for organic compounds and isotopic abundances, which can be used in measurement devices, instruments, scientific instruments, etc., and can solve the problems of cumbersome operations, long time consumption, and inaccurate isotopic abundance results in the transformation process.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
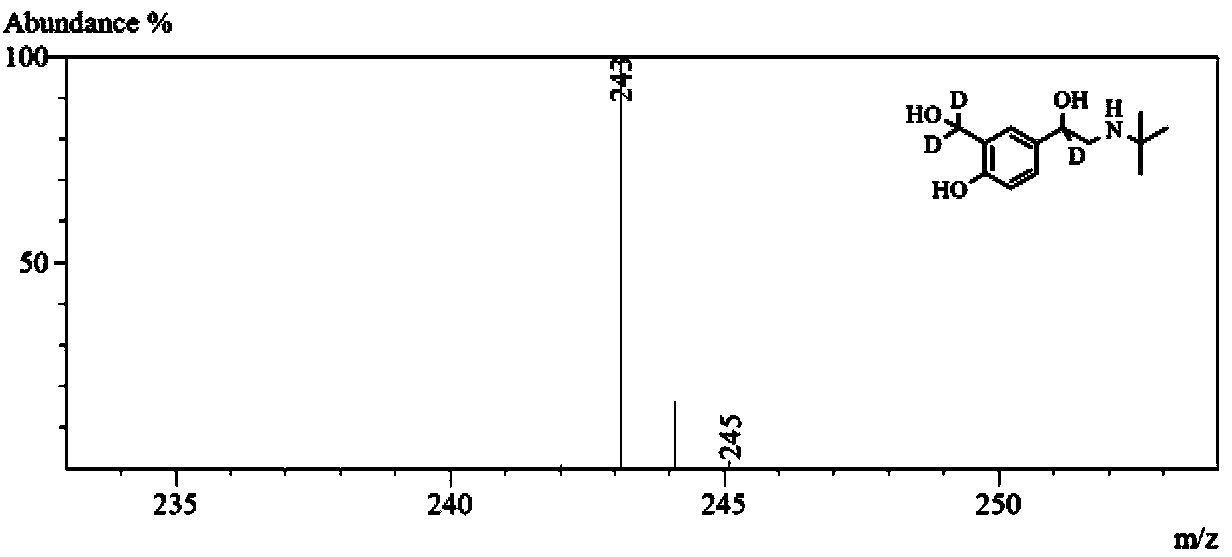
[0070] Salbutamol-D 3 D isotope label abundance analysis
[0071] Sample pretreatment, chromatographic conditions and mass spectrometry parameters are as described in the summary of the invention. Salbutamol-D 3 The structural formula and mass spectrum of figure 1 shown.
[0072] Target compound albuterol-D 3 The molecular formula is C 13 h 18 NO 3 D. 3 , after electrospray ionization will form [M+H] + molecular ions. Through the "mass cluster" classification method, the target compound ions can be divided into [C 13 h 19 NO 3 (H 3 D. 0 )] + , [C 13 h 19 NO 3 (H 2 D. 1 )] + , [C 13 h 19 NO 3 (H 1 D. 2 )] + and [C 13 h 19 NO 3 (H 0 D. 3 )] + Four "mass clusters", as shown in Table 2. Their mass distributions are similar to those of the non-marked sites "C 13 h 19 NO 3 "The natural isotope distribution is the same, according to the calculation results of the isotope abundance calculator, the isotope distribution ratio is 0.858:0.127:0.014:0.0...
Embodiment 2
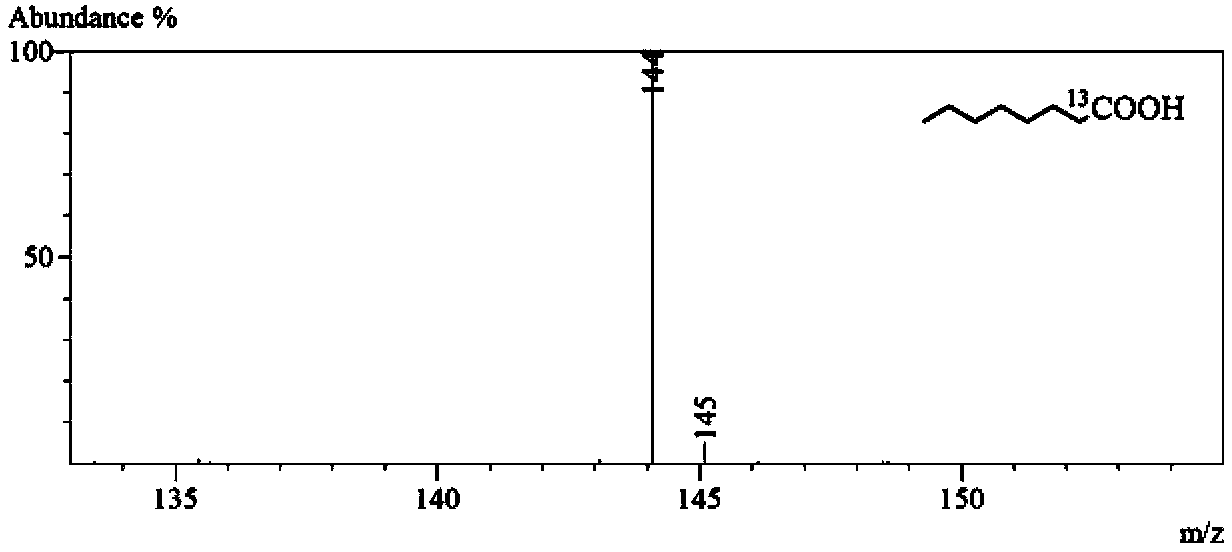
[0088] Caprylic acid-1- 13 Isotopic abundance analysis of C
[0089] Sample pretreatment, chromatographic conditions and mass spectrometry parameters are as described in the summary of the invention. For octanoic acid-1- 13 C, due to its better negative ion signal response, the negative ion scanning mode is used. Caprylic acid-1- 13 The structural formula and mass spectrum of C are as follows figure 2 shown.
[0090] The target compound octanoic acid-1- 13 The molecular formula of C is C 7 h 16 o 2 13 C, [M-H] will be formed after electrospray ionization - molecular ions. Through the "mass cluster" classification method, the target compound ions can be divided into [C 7 h 15O2 ( 12 C 1 13 C 0 )] - and [C 7 h 15 o 2 ( 12 C 0 13 C 1 )] - Two "mass clusters", as shown in Table 4. Their mass distributions are similar to those of the non-marked sites "C 7 h 15 o 2 "The natural isotope distribution is the same, according to the calculation results of t...
Embodiment 3
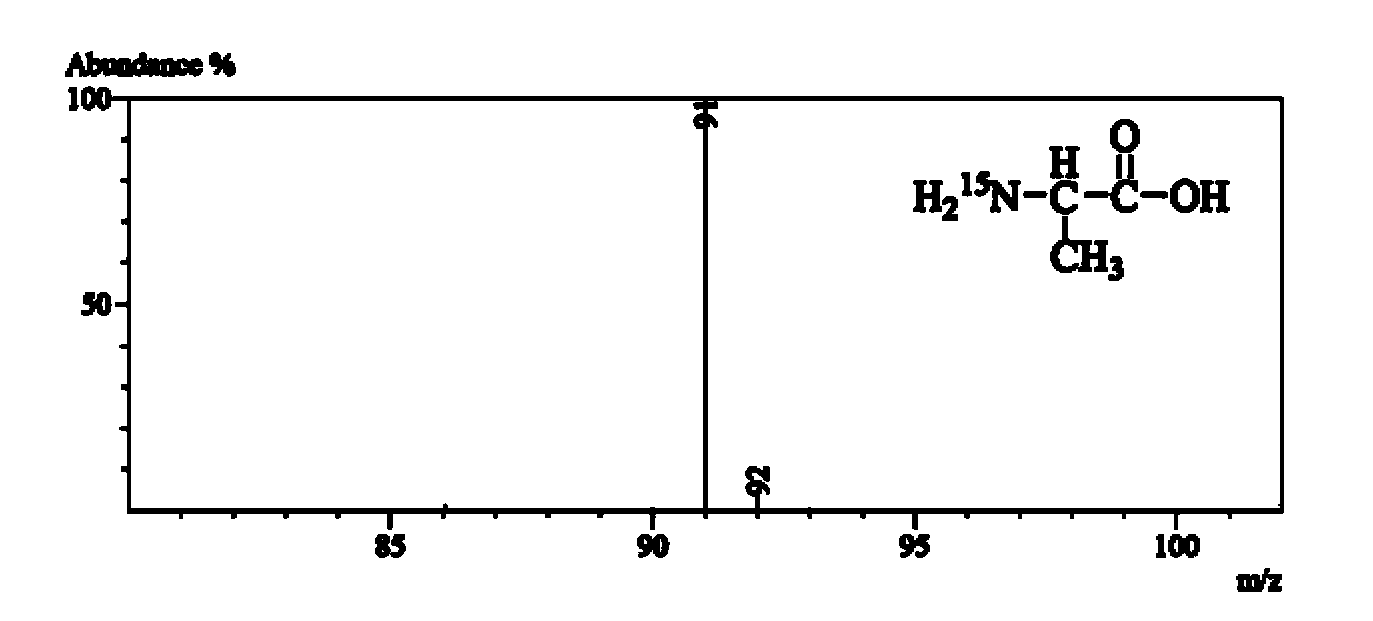
[0102] L-alanine- 15 N isotopic abundance analysis
[0103] Sample pretreatment, chromatographic conditions and mass spectrometry parameters are as described in the summary of the invention. L-alanine- 15 The structural formula and mass spectrum of N are as follows image 3 shown.
[0104] Target compound L-alanine- 15 The molecular formula of N is C 3 h 7 o 2 15 N, will form [M+H] after electrospray ionization + molecular ions. Through the "mass cluster" classification method, the target compound ions can be divided into [C 3 h 8 o 2 ( 14 N 1 15 N 0 )]+ and [C 3 h 8 o 2 ( 14 N 0 15 N 1 )] + Two "mass clusters", as shown in Table 6. Their mass distributions are similar to those of the non-marked sites "C 3 h 8 o 2 "The natural isotope distribution is the same, according to the calculation results of the isotope abundance calculator, the isotope distribution ratio is 0.963:0.033:0.004.
[0105] Table 6 According to the "mass cluster" to the target c...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com