Injectable bone cement containing strontium and preparation method of bone cement
A bone cement and strontium source technology, applied in the field of strontium-containing injectable bone cement and its preparation, can solve the problems of non-absorption and degradation, weak integration with bone, and no osteogenesis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
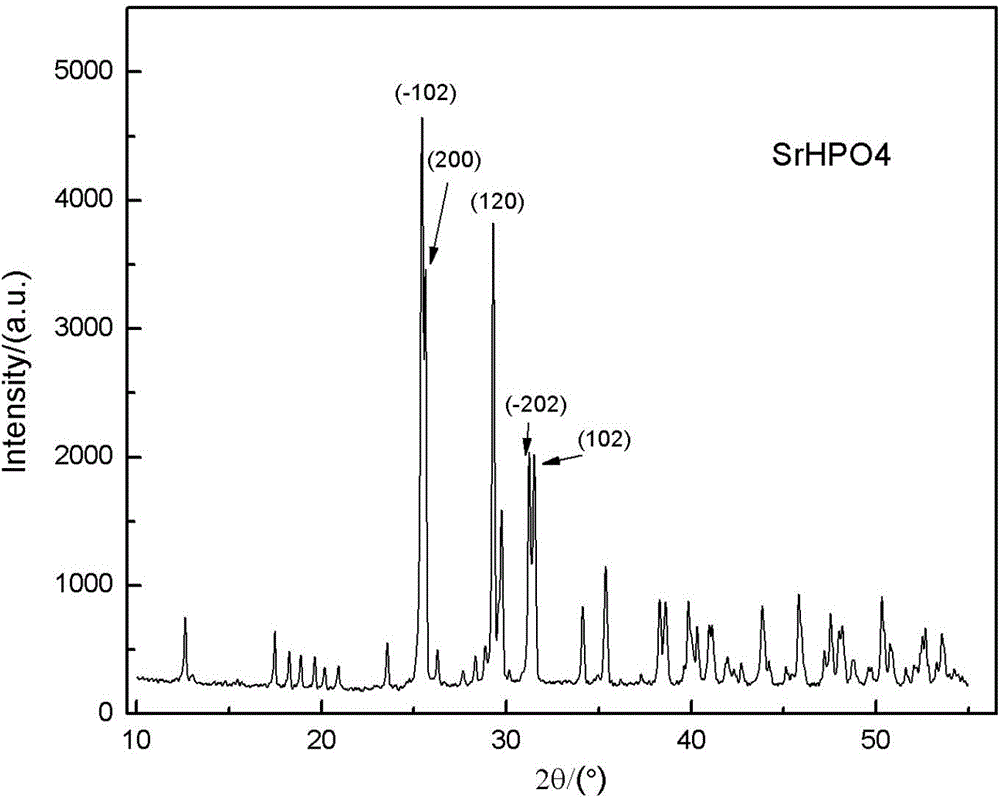
[0035] (1) The preparation method of strontium hydrogen phosphate powder: configure 200ml of 0.5mol / L strontium nitrate solution, and add 0.6mol urea into it and stir to dissolve to obtain A solution; configure 200ml of 0.5mol / L diammonium hydrogenphosphate solution, Obtain B solution; Pour B solution into A solution, stir and mix, the solution becomes a turbid suspension, add concentrated nitric acid (65-68%), adjust the pH value to 2, the solution becomes clear; put the clear solution into In a water bath at 80°C, react at a constant temperature for 4 hours, pour out the supernatant, wash repeatedly, filter the white precipitate, dry and grind at a constant temperature in an oven at 100°C to obtain a powder product. The prepared product was characterized by XRD, as attached figure 1 As shown, the absorption peak corresponds well to the standard peak (PDF33-1335), and it can be determined that the prepared product is strontium hydrogen phosphate.
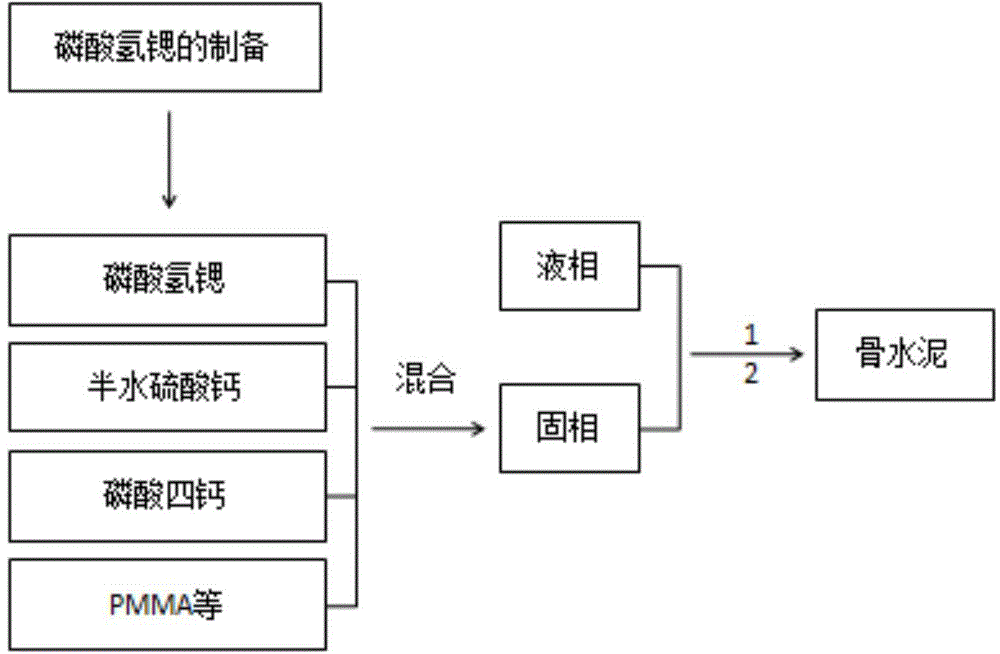
[0036] (2) The preparation...
Embodiment 2
[0039](1) The preparation method of strontium hydrogen phosphate powder: configure 200ml of 0.3mol / L strontium nitrate solution, add 0.3mol urea into it and stir to dissolve to obtain A solution; configure 200ml of 0.3mol / L diammonium hydrogenphosphate solution, Obtain B solution; Pour B solution into A solution, stir and mix, the solution becomes a turbid suspension, add concentrated nitric acid (65-68%), adjust the pH value to 2, the solution becomes clear; put the clear solution into In a water bath at 90°C, react at a constant temperature for 3 hours, pour out the supernatant, wash repeatedly, filter the white precipitate with suction, dry and grind at a constant temperature in an oven at 85°C to obtain a powder product. According to Example 1, the product was subjected to phase analysis.
[0040] (2) The preparation method of bone cement: at room temperature, according to proportioning, take by weighing 5.75gPMMA (57.5%) respectively, 0.25gBPO (2.5%), CSH (2.1g, 60%) of 3...
Embodiment 3
[0042] (1) The preparation method of strontium hydrogen phosphate powder: configure 200ml of 0.05mol / L strontium nitrate solution, and add 0.04mol urea into it and stir to dissolve to obtain A solution; configure 200ml of 0.05mol / L diammonium hydrogenphosphate solution, Obtain B solution; Pour B solution into A solution, stir and mix, the solution becomes a turbid suspension, add concentrated nitric acid (65-68%), adjust the pH value to 3, the solution becomes clear; put the clear solution into In a water bath at 100°C, react at a constant temperature for 2 hours, pour out the supernatant, wash repeatedly, filter the white precipitate with suction, dry and grind at a constant temperature in an oven at 85°C to obtain a powder product. According to Example 1, the product was subjected to phase analysis.
[0043] (2) The preparation method of bone cement: at room temperature, according to proportioning, take by weighing 4.95gPMMA (49.5%) respectively, 0.05gBPO (0.5%), CSH (1.35g,...
PUM
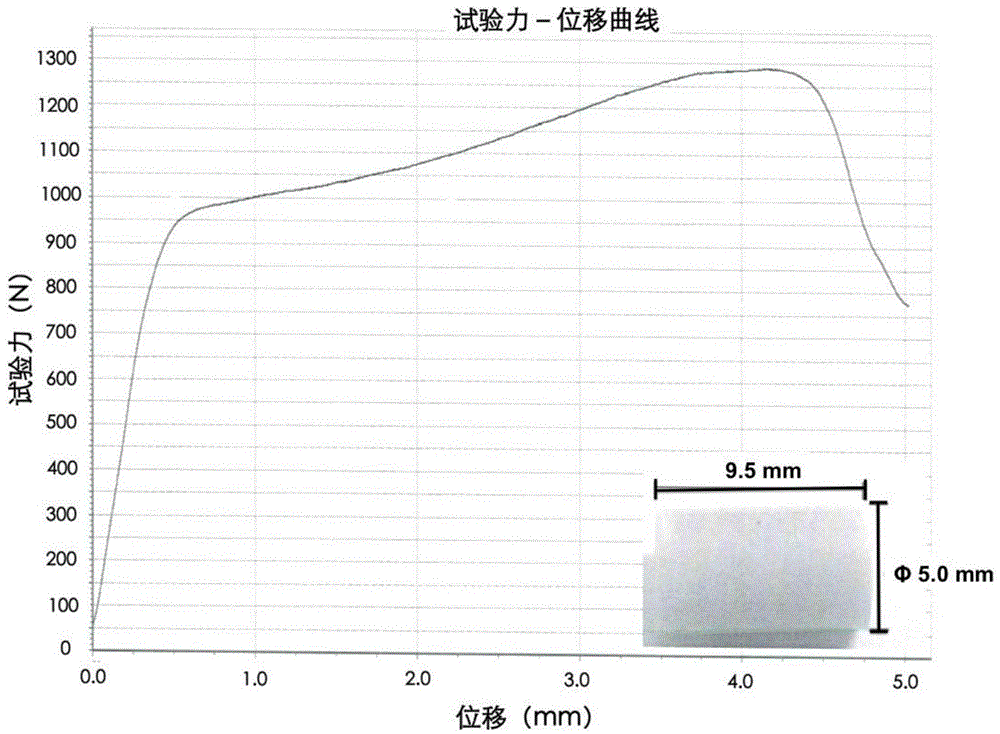
| Property | Measurement | Unit |
|---|---|---|
| compressive strength | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
| length | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com