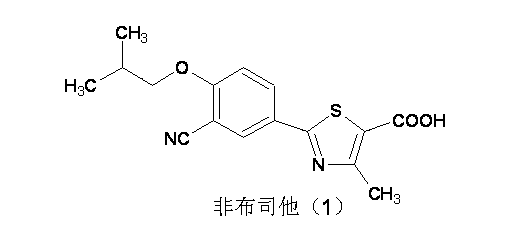
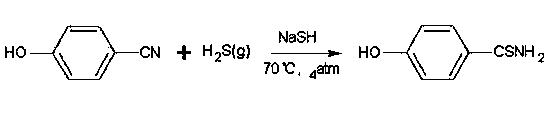Preparation method of 3,4-substitituted thiobenzamide and application of 3,4-substitituted thiobenzamide in febuxostat synthesis
A technology of thiobenzamide and thioacetamide, applied in the direction of organic chemistry, etc., can solve the problems of large discharge pollution of three wastes, complicated operation and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
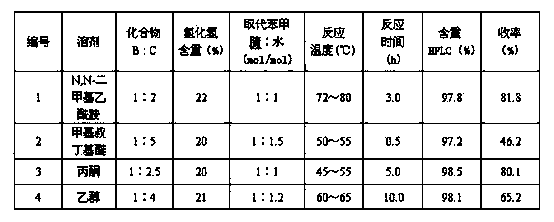
[0023] Table 1 The research results of changing the reaction solvent and temperature and other factors
[0024]
[0025] The 4-hydroxythiobenzamide obtained by the above method is used as a raw material to synthesize Febuxostat according to the methods of patents JP6-329647 / 1994 and JP10-45733 / 1998.
[0026] Example 2
Embodiment 2
[0028] Table 2 The research results of changing the reaction solvent and temperature and other factors
[0029]
[0030] Using the 4-hydroxy-3-nitrothiobenzamide obtained by the above method as a raw material, Febuxostat was synthesized by referring to the method of patent EP0513379 / 1992.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com