Compound amoxicillin powder and production process thereof
A technology of amoxicillin powder and production process, which is applied in the field of veterinary medicine, can solve problems such as content drop, humid environment, caking, etc., and achieve the effect of high production efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
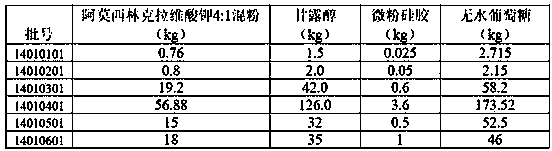
[0019] In this example, six batches of samples were prepared according to the ratio in Table 1.
[0020] Table 1 Weight ratio of each batch
[0021]
[0022] Among them, the production process of the 14010101 batch is: control the production environment temperature below 25°C and relative humidity below 20%, and perform the following operations:
[0023] (1) Crush mannitol, finely powdered silica gel and anhydrous glucose separately, pass through an 80-mesh sieve, mix in a drying mixer at 80±5°C for 1 hour, and cool to room temperature naturally;
[0024] (2) Add amoxicillin and clavulanate potassium mixed powder 4:1 into the drying mixer and continue mixing for 20 minutes;
[0025] (3) After vacuum packaging in aluminum foil bags, then packaging in plastic-aluminum composite bags.
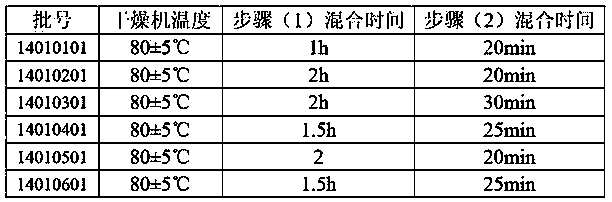
[0026] The production process of the remaining batches is the same as the steps, and the differences are shown in Table 2.
[0027] Table 2 Production process parameters of each batch
[0028]
Embodiment 2
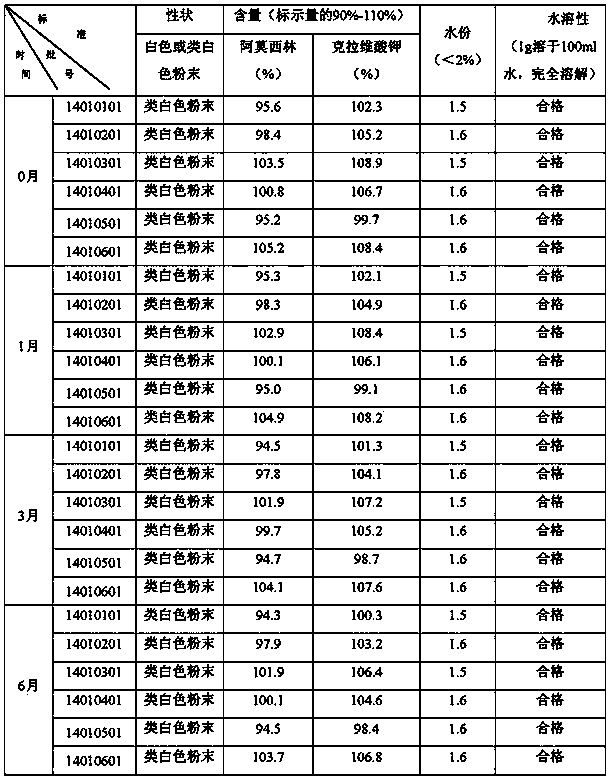
[0030] In order to investigate the feasibility of the present invention, this example extracts 6 batches of samples in Example 1 for stability investigation. The method is: take different batches of samples and place them under accelerated conditions of 42°C and 75% humidity. A 6-month stability investigation was carried out, and samples were taken at the 0th, 1, 3, and 6th months according to the first volume of the "National Standard for Veterinary Drugs" (Chemical Drugs, Chinese Medicine Volume), and the test results See Table 3.
[0031] Table 3 Accelerated test results of different batches of compound amoxicillin powder
[0032]
[0033] Tests confirmed that the compound amoxicillin powder of the present invention is under the control of the new process conditions and verified by accelerated tests, the traits, the content of amoxicillin and clavulanic acid have not changed significantly, and all the test items meet the "National Standard for Veterinary Drugs" (Chemical The pr...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com