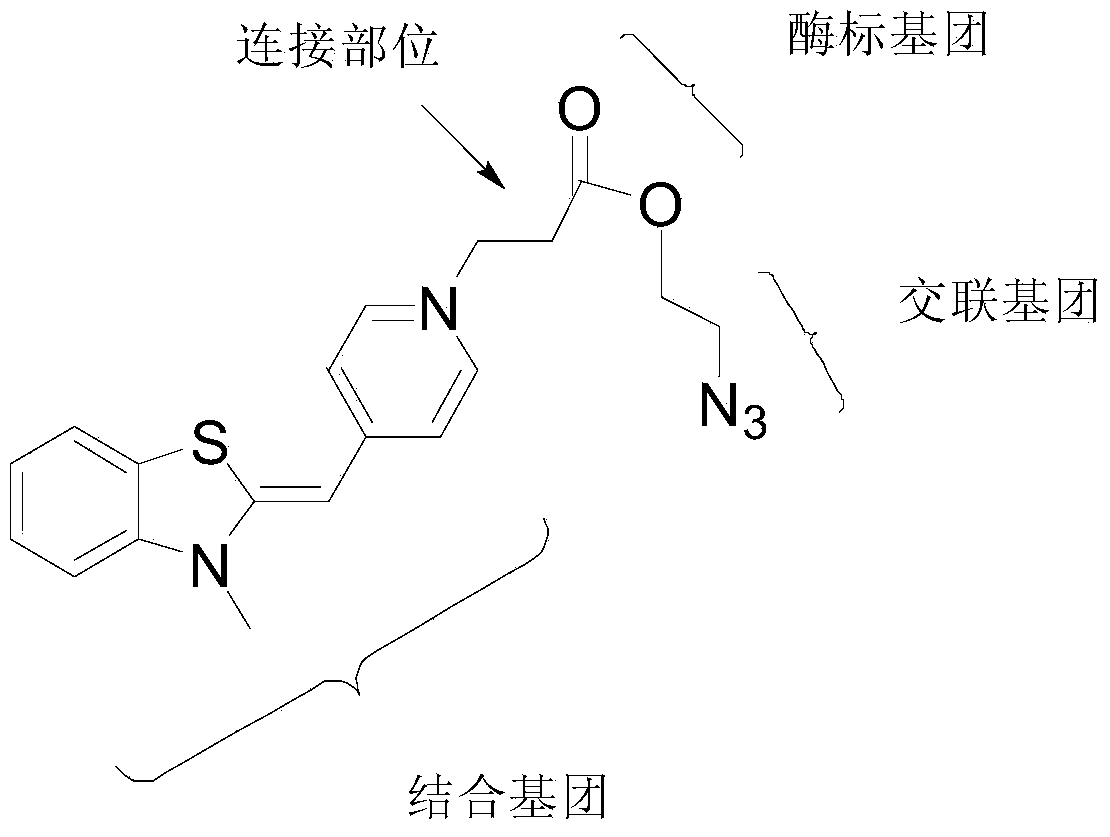
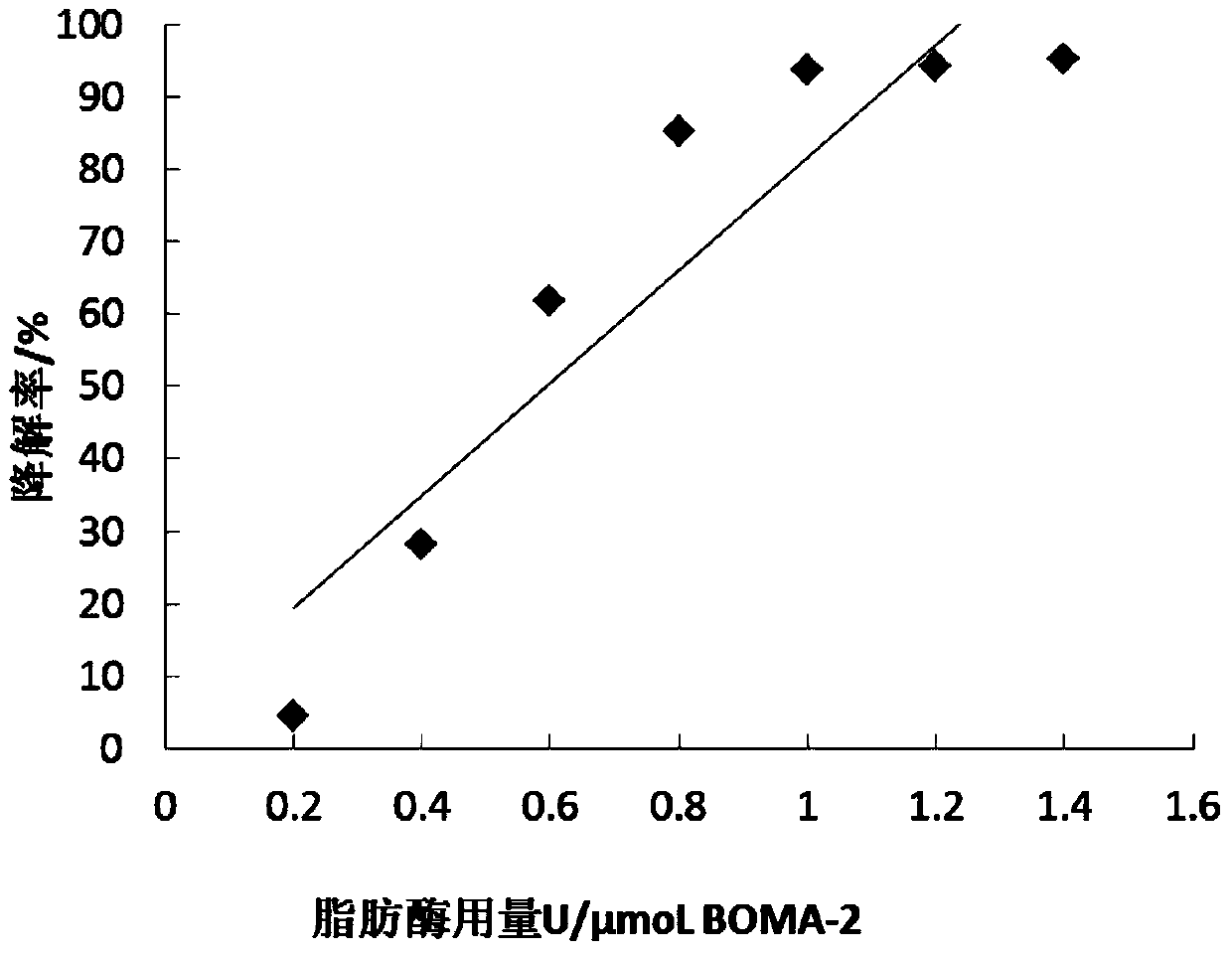
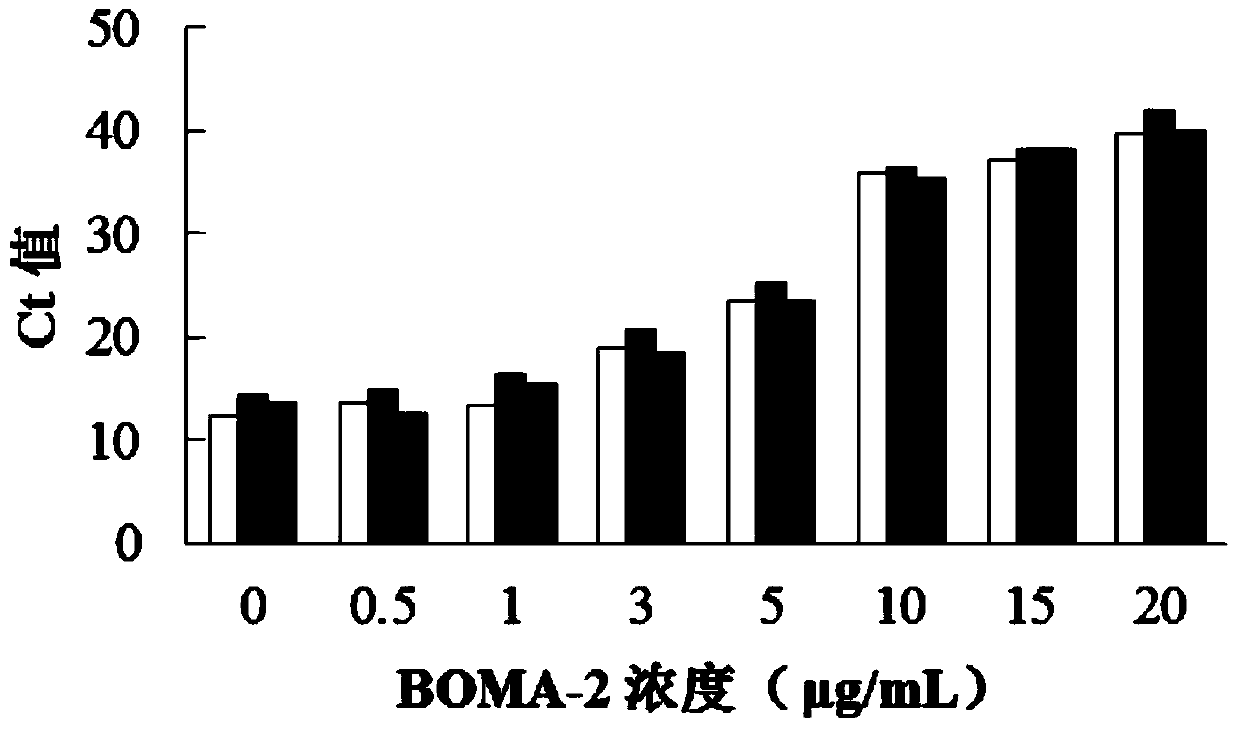
Thiazole orange cyanine dye molecule and application thereof
A technology of orange-like cyanine and molecule, which is applied in thiazole orange-like cyanine dye molecule and its application field, can solve problems such as membrane damage, and achieve the effects of low preparation cost, reduced synthesis cost, and high esterase degradation rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] A kind of thiazole orange cyanine dye molecule (BOMA-2), its preparation method is as follows:
[0036] The overall chemical reaction formula is
[0037]
[0038] Concrete preparation steps are as follows:
[0039] Preparation of compound 2:
[0040]
[0041] 1.50 g of compound 1 was mixed with 2.00 g of bromoacetic acid, and heated at 120° C. for 5 hours. After the reaction temperature was lowered to room temperature, the obtained brown solid was dissolved in methanol and concentrated in vacuo. The concentrated material was dissolved in 20 mL of dichloromethane and cooled to 0°C. 40 mL of acetone was slowly added dropwise, and the solid was collected by filtration. After washing three times with 15 mL of acetone, the obtained crude material was resuspended in 20 mL of dichloromethane and stirred for 30 minutes. The solid was collected by filtration and washed three times with 15 mL of dichloromethane to obtain 1.32 g of a light gray solid with a yield of 39%...
Embodiment 2
[0073] A kind of thiazole orange cyanine dye molecule (BOMA-5), its preparation method is as follows:
[0074] Preparation of compound 2:
[0075]
[0076] 1.50 g of compound 1 was mixed with 2.26 g of 6-bromohexanoic acid, and heated at 120° C. for 5 hours. After the reaction temperature was lowered to room temperature, the obtained brown solid was dissolved in methanol and concentrated in vacuo. The concentrated material was dissolved in 20 mL of dichloromethane and cooled to 0°C. 40 mL of acetone was slowly added dropwise, and the solid was collected by filtration. After washing three times with 15 mL of acetone, the obtained crude material was resuspended in 20 mL of dichloromethane and stirred for 30 minutes. The solid was collected by filtration and washed three times with 15 mL of dichloromethane to obtain 1.44 g of a light gray solid with a yield of 41%.
[0077] Preparation of compound 3:
[0078]
[0079] After 1.4 g of compound 2 was stirred evenly with d...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com