Polyalkyloxy substituted 2,3-dicarboxylate benzophenanthrene and its preparation method
A technology of polyalkoxy and dicarboxylates, which is applied in the preparation of carboxylic acid esters, the preparation of organic compounds, chemical instruments and methods, etc., and can solve the problems of long reaction steps, low yields, and difficulties
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
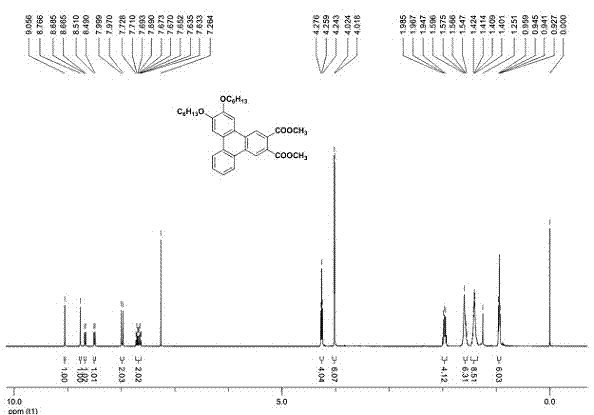
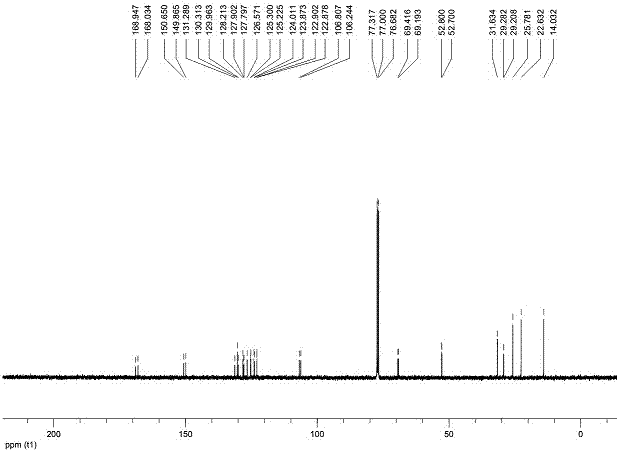
[0041] The compound provided in this example is a compound of general formula (I), wherein R 1 , R 2 for-OC 6 h 13 , R 3 , R 4 , R 5 , n=1. Its preparation reaction is as follows:
[0042]
[0043] In the reaction formula, toluene is toluene; DMAD is dimethyl butynedicarboxylate.
[0044] Concrete preparation steps are as follows:
[0045] Add 1 mole of diaryl acetylene, 0.1 mole of cuprous iodide and 0.2 mole of Grubbs-2 into the reaction tube, close the system, and use toluene as solvent under ethylene atmosphere, at 80 o Heated and stirred in an oil bath of C for 24 hours, cooled slightly, added 7.0 moles of dimethyl butynedicarboxylate (DMAD), at 100 o Continue heating and stirring in an oil bath of C for 24 hours. The system was cooled to room temperature, the reaction solution was passed through a silica short column, washed with dichloromethane to remove insoluble matter, and the filtrate solvent was removed by rotary evaporation, and the obtained solid ...
Embodiment 2
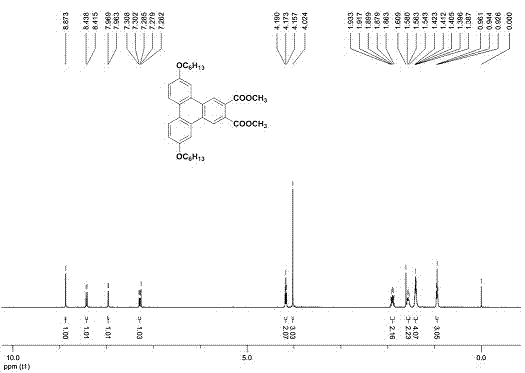
[0052] The compound provided in this example is a compound of general formula (I), wherein R 1 , R 5 for-OC 6 h 13 , R 2 , R 3 , R 4 is H, n=1. Its preparation reaction is as follows:
[0053]
[0054] In the reaction formula, toluene is toluene; DMAD is dimethyl butynedicarboxylate; DDQ / MeSO 3 H is 2,3-dichloro-5,6-dicyano-p-benzoquinone / methanesulfonic acid.
[0055] Concrete preparation steps are as follows:
[0056] Add 1 mole of diaryl acetylene, 0.1 mole of cuprous iodide and 0.2 mole of Grubbs-2 into the reaction tube, close the system, and use toluene as solvent under ethylene atmosphere, at 80 o Heated and stirred in an oil bath of C for 24 hours, cooled slightly, added 7.0 moles of dimethyl butynedicarboxylate (DMAD), at 100 o Continue heating and stirring in an oil bath of C for 24 hours. The system was cooled to room temperature, and the reaction liquid was passed through a short column of silica, washed with dichloromethane to remove insoluble mat...
Embodiment 3
[0063] The compound provided in this example is a compound of general formula (I), wherein R 1 , R 2 for-oc 6 h 13 , R 3 , R 5 for H, R 4 for-OCH 3 , n=1. Its preparation reaction is as follows:
[0064]
[0065] In the reaction formula, toluene is toluene; DMAD is dimethyl butynedicarboxylate.
[0066] Concrete preparation steps are as follows:
[0067] Add 1 mole of diaryl acetylene, 0.05 mole of cuprous iodide and 0.1 mole of Grubbs-2 into the reaction tube, use toluene as solvent, under ethylene atmosphere at 80 o Heated and stirred in an oil bath of C for 24 hours, cooled slightly, added 5.0 moles of dimethyl butynedicarboxylate (DMAD), at 100 o Continue heating and stirring in an oil bath of C for 24 hours. The system was cooled to room temperature, the reaction solution was passed through a short silica column, washed with dichloromethane to remove insoluble matter, and the filtrate solvent was removed by rotary evaporation, and the obtained solid was add...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com