A pharmaceutical composition comprising butylphthalide and borneol and applications thereof
A technology of composition and butylphthalide, which is applied in the direction of drug combination, medical preparations containing active ingredients, active ingredients of hydroxyl compounds, etc., can solve problems such as the impact of drug safety, reduce drug dosage, reduce adverse reactions, reduce The effect of the effective dose
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] Embodiment 1: the preparation of injection medicine solution
[0028] (1) Preparation of 30% HP-β-CD
[0029] Take 600 g of HP-β-CD (hydroxypropyl-β-cyclodextrin), add 1200 mL of water for injection, stir to dissolve, and finally dilute to 2000 mL with water for injection, which is 30% HP-β-CD;
[0030] (2) Preparation of butylphthalide solution for injection
[0031] Weigh 4g each of s-butylphthalide and levo-butylphthalide respectively, then add 600mL 30% HP-β-CD respectively, stir until the solution is clear, and obtain it;
[0032] (3) Preparation of borneol solution for injection
[0033] Take 1 g of natural borneol, add 20mL of 95% ethanol, mix well, add 280mL of 30% HP-β-CD, stir until the solution is clear, and obtain;
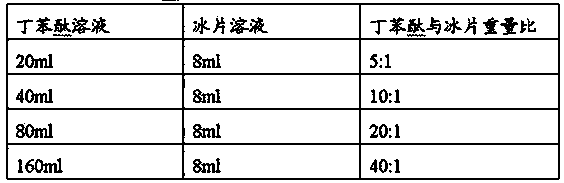
[0034] (4) Preparation of butylphthalide + borneol solution for injection
[0035] Take the butylphthalide solution and borneol solution in a given ratio, mix them well, and get that, the mixing ratio is as follows:
[0036]
[0037] The ...
Embodiment 2
[0038] Embodiment 2: Preparation of oral medicinal liquid oil
[0039] (1) Preparation of Butylphthalide Liquid Oil
[0040] Respectively weigh 25g of butylphthalide and 25g of butylphthalide respectively, then add them into 225g of soybean oil respectively, and stir well to obtain the product;
[0041] (2) Preparation of borneol liquid oil
[0042] Take 10g of natural borneol, add it into 90g of soybean oil, stir and dissolve until the solution is clear, and you get it;
[0043] (3) Preparation of butylphthalide + borneol liquid oil
[0044] Take the butylphthalide medicinal liquid oil and borneol medicinal liquid oil in a given ratio, and mix them evenly to get the product. The mixing ratio is as follows:
[0045]
[0046] Above-mentioned medicinal liquid oil also can adopt conventional method as CN1623542A discloses the preparation method of soft capsule, is compressed into soft capsule.
Embodiment 3
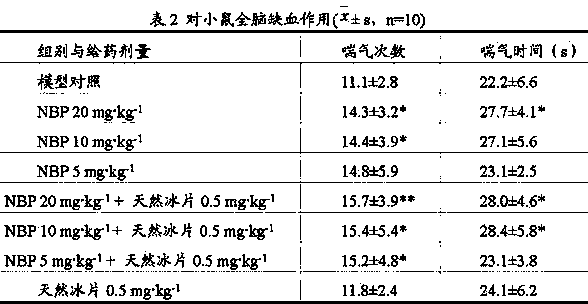
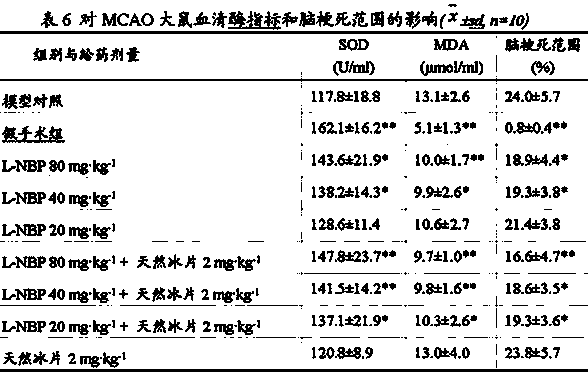
[0047] Embodiment 3: Mouse global ischemia test (borneol dosage investigation)
[0048] 1 Animal experiment materials
[0049] 1.1 Drugs Test drugs: racemic butylphthalide (NBP) solution for injection prepared in Example 1, natural borneol solution for injection, racemic butylphthalide + natural borneol solution for injection, model control is 30% HP-β-CD .
[0050] 1.2 Animals SPF grade KM mice, male, 18-22 grams, provided by the Experimental Animal Center of Hebei Medical University; the experimental animal production license number is SCXK (Ji) 2008-1-003. Experimental animal use license number: SYXK (Ji) 2011-0059.
[0051] 1.3 Data processing The data were analyzed by variance analysis and intergroup test by SPSS11.5 statistical software.
[0052] 2 Mice global cerebral ischemia test
[0053] 2.1 Animal grouping and administration: 100 Kunming mice were randomly divided into 10 groups, namely the model control group (30% HP-β-CD), the NBP group (10 mg?kg -1 ), natu...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com