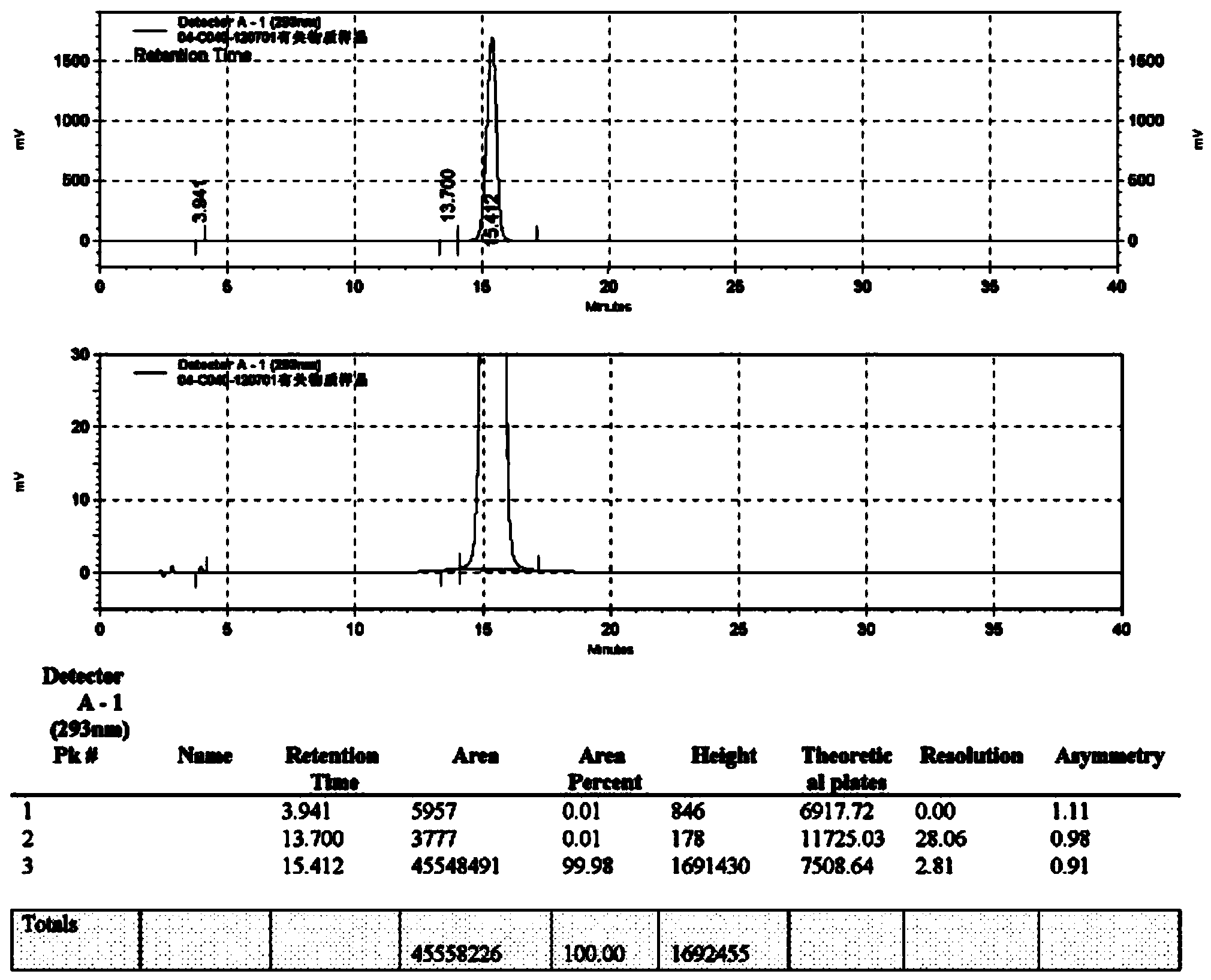
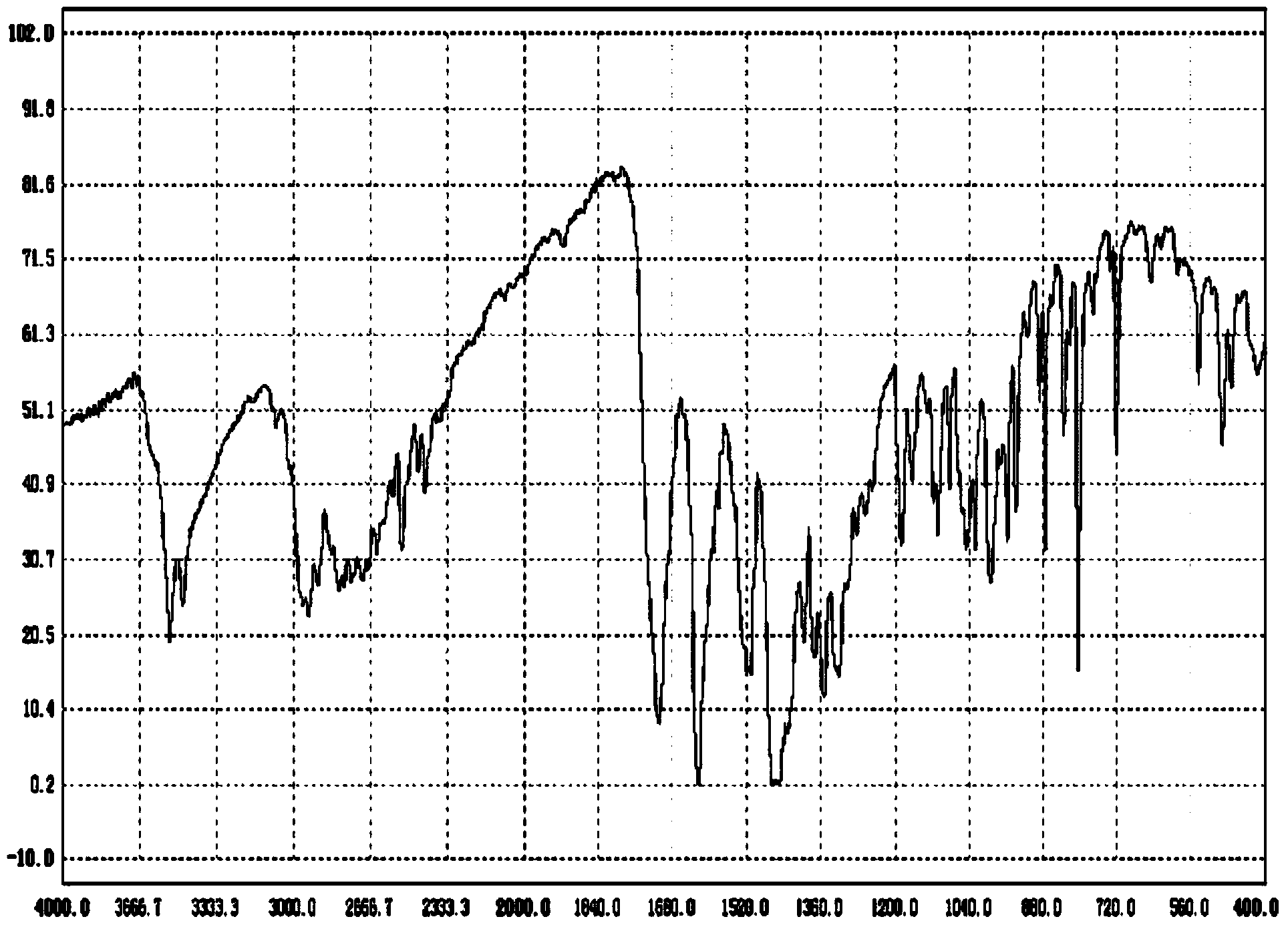
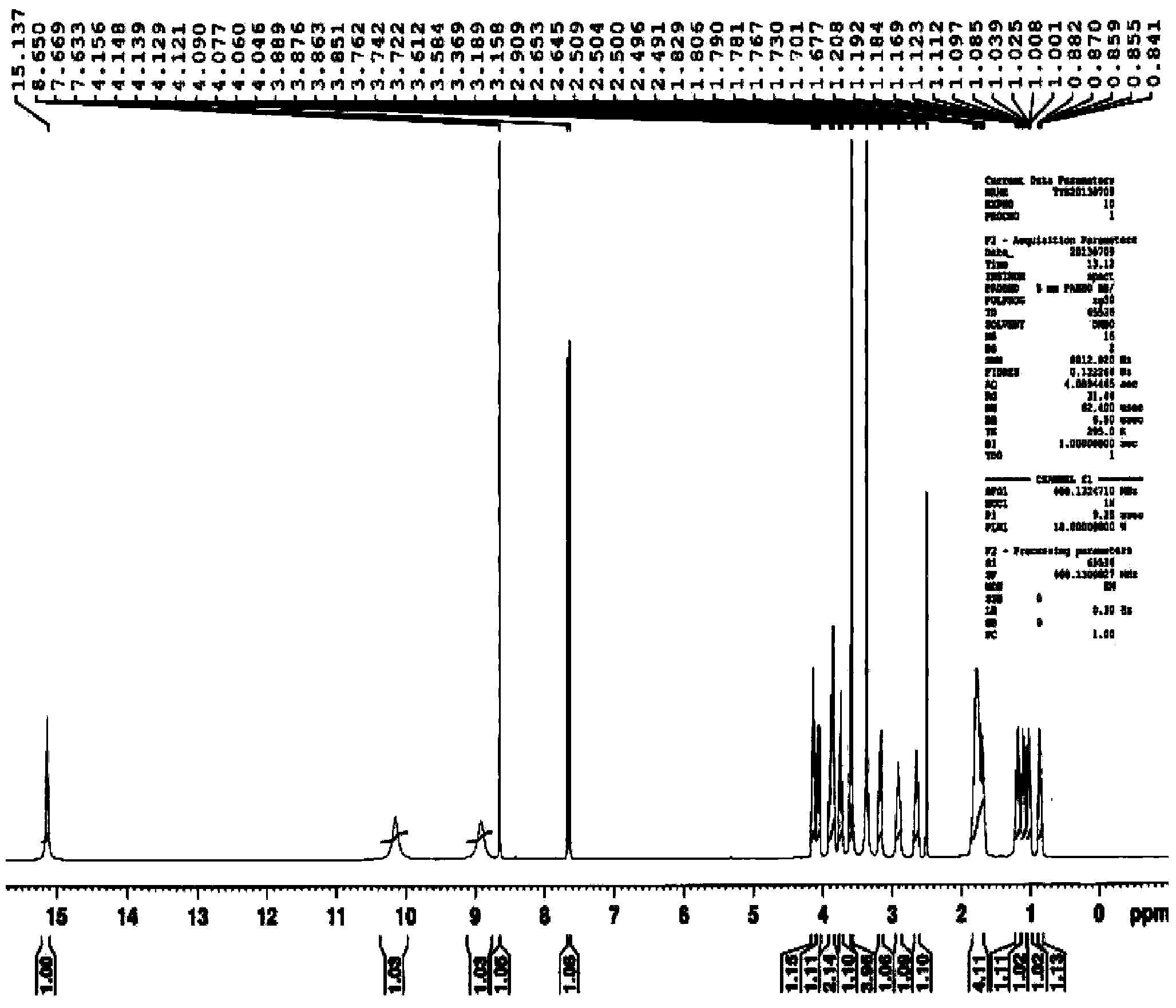
Synthetic method of moxifloxacin hydrochloride
A technology of moxifloxacin hydrochloride and a synthesis method, applied in the direction of organic chemistry and the like, can solve the problems of increased amount of impurities and high energy consumption, and achieve the effects of reducing the generation of impurities, saving energy, and mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] The preparation of embodiment 1 main ring chelate
[0034] Put 240g of acetic anhydride into a small reactor and heat to 70°C, slowly add 36g of boric acid between 70-90°C, heat up and reflux for 1 hour, then cool to 70°C, add 120g of 1-cyclopropyl-6,7-difluoro under stirring -Ethyl 1,4-dihydro-8-methoxy-4-oxo-3-quinolinecarboxylate, heat up to 100-105°C for 1 hour, cool to 0°C, add 480ml of ice water slowly , then add 480ml of cold water at 0-5°C, keep at 0-5°C for 2h, the product precipitates, filters, washes with 480ml of water, and vacuum-dries at 40°C until the water content is 2%, to obtain 152.4g of the main ring chelate.
Embodiment 2
[0035] The preparation of embodiment 2 main ring chelates
[0036] Put 120g of acetic anhydride into the reaction flask and heat to 70°C, slowly add 12g of boric acid between 70-90°C, heat up and reflux for 1h, then cool to 70°C, add 30g of 1-cyclopropyl-6,7-difluoro- Ethyl 1,4-dihydro-8-methoxy-4-oxo-3-quinolinecarboxylate, heat up to 100-105°C for 1 hour, cool to 0°C, add 210ml of ice water slowly, Then add 210ml of cold water at 0-5°C, keep it at 0-5°C for 2 hours, the product precipitates, filters, washes with 210ml of water, and vacuum-dries at 50°C until the water content is 2.6%, to obtain 38.2g of the main ring chelate.
Embodiment 3
[0037] The preparation of embodiment 3 main ring chelates
[0038] Put 100g of acetic anhydride into the reaction flask and heat to 70°C, slowly add 10g of boric acid between 70-90°C, heat up and reflux for 1h, then cool to 70°C, add 20g of 1-cyclopropyl-6,7-difluoro- Ethyl 1,4-dihydro-8-methoxy-4-oxo-3-quinolinecarboxylate, heat up to 100-105°C for 1 hour, cool to 0°C, add 280ml of ice water slowly, Then add 280ml of cold water at 0-5°C, keep it at 0-5°C for 2 hours, the product precipitates, filters, washes with 280ml of water, and vacuum-dries at 50°C until the water content is 3.9%, to obtain 26.0g of the main ring chelate.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com