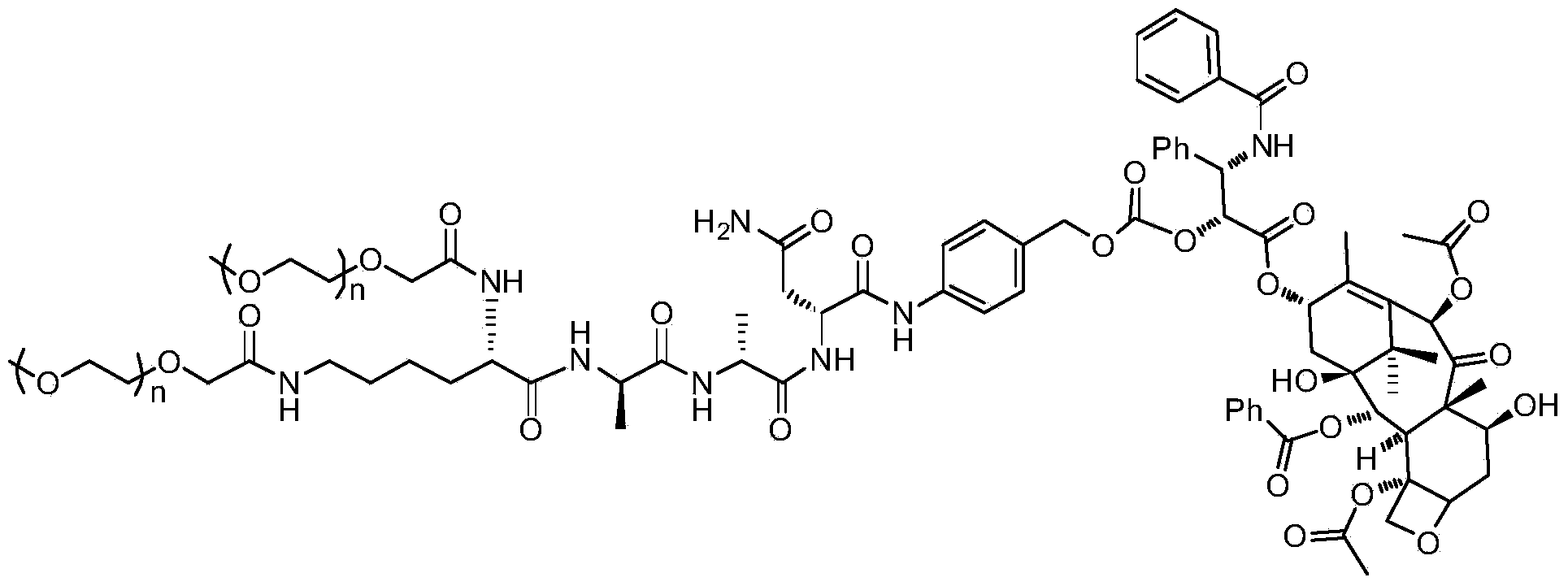
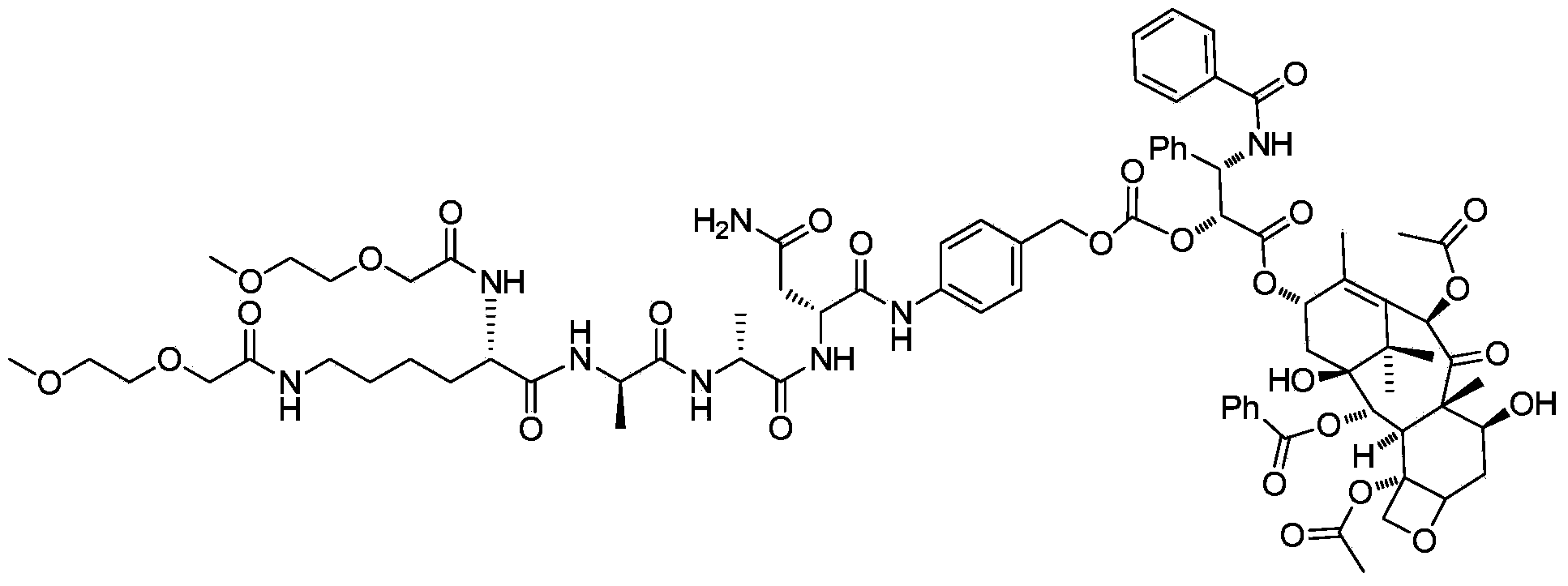
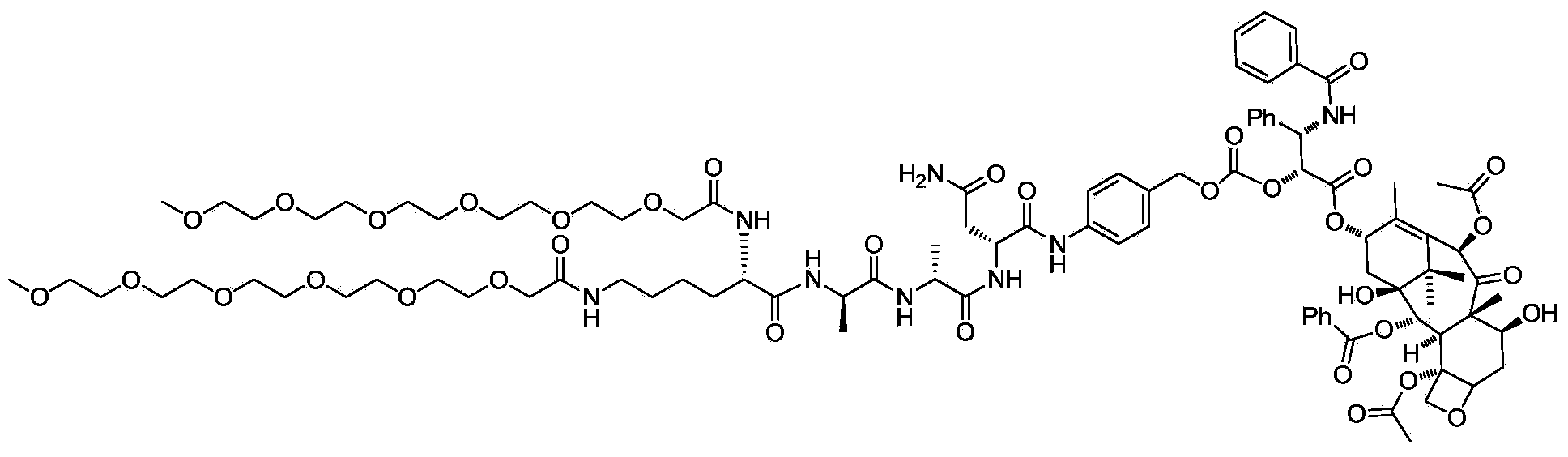
Water soluble targeting-activated taxol derivatives as well as preparation method and use thereof
A paclitaxel derivative, water-soluble technology, applied in the field of drugs and anti-tumor drugs, can solve the problems of neutropenia, allergic reactions, toxic and side effects, etc., and achieve the effects of reduced toxicity, weakened toxicity, blood stability and low toxicity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] Example 1: Synthesis of water-soluble targeted-activated paclitaxel
[0028] 1) Synthesis of two (2-methoxyethoxyacetyl)-L-lysine ethyl ester (I)
[0029] Dissolve 2-(2-methoxyethoxy)acetic acid (161mg, 1.2mmol) in N,N-dimethylformamide (10mL), and after cooling in an ice bath, add 2-(7- Nitrobenzotriazole)-N,N,N',N'-tetramethyluronium hexafluorophosphate (462mg, 1.2mmol), N,N-diisopropylethylamine (313mg, 2.4mmol) and L-lysine ethyl ester dihydrochloride (100mg, 0.4mmol), after addition, stirred at room temperature overnight, evaporated the solvent under reduced pressure, and purified the crude product by a reverse-phase preparative column to obtain I (128mg, yield: 77.8%) .
[0030] 2) Synthesis of two (2-methoxyethoxyacetyl)-L-lysine (II)
[0031] Dissolve bis(2-methoxyethoxyacetyl)-L-lysine (122mg, 0.3mmol) in tetrahydrofuran (15mL), cool to 0°C and add dropwise lithium hydroxide (39mg, 0.9mmol) Aqueous solution (5 mL), stirred at room temperature for 2 hours. ...
Embodiment 2
[0047] Example 2 Comparison of the performance of the water-soluble target-activated paclitaxel derivatives prepared in the examples of the present invention and the reference compound
[0048] (1) Sample processing
[0049] The water-soluble target-activated paclitaxel derivatives prepared in the examples of the present invention, compounds S1, S2, S3 and S4 (prepared in Examples 10-11) and the above-mentioned reference compounds C1, C2, C3, C4, C5, C6. After freeze-drying (-70°C), subpackage in a sterile room. Before animal experiments, S1, S2, S3 and S4 can be reconstituted in the sterile room according to the following two solvent schemes: solvent 1 (water for injection) or solvent 2 (45% alcohol, 55% water for injection). S1, S2, S3 and S4 can be completely dissolved in solvent 1 and solvent 2, and the reconstitution concentration can reach 10 mg / ml, and then diluted with water for injection to the required concentration, while the comparison compounds (C1, C2, C3, C4, ...
Embodiment 3
[0058] Example 3 S1, S2, S3 and S4 (prepared in Examples 10-11) content determination method and content range.
[0059] S1, S2, S3 and S4 use analytical HPLC (Agilent 1220 (Agilent 1220series), C8 column 5μm, 4.6mm ID x250mm, mobile phase is 0-95% acetonitrile (ACN) with a purity of 95%-99%).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com