Preparation method of waterborne non-isocyanate polyurethane modified polyester
A water-based non-isocyanate, polyurethane modification technology, applied in the direction of coating, etc., can solve the problem that water-based non-isocyanate polyurethane modified polyester has not yet been developed
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0040] Preparation method of water-based non-isocyanate polyurethane modified polyester
[0041] In a first aspect of the present invention, a kind of preparation method of the polyester of waterborne non-isocyanate polyurethane modification is provided, and the method may further comprise the steps:
[0042] (1) preparation of unsaturated prepolymer containing urethane bond;
[0043] (2) Preparation of polyester containing unsaturated double bonds;
[0044] (3) the graft polymerization of the polyester containing unsaturated double bond and prepolymer of gained;
[0045] (4) Dispersion of the grafted polymer.
[0046] (1) Preparation of unsaturated prepolymers containing urethane bonds
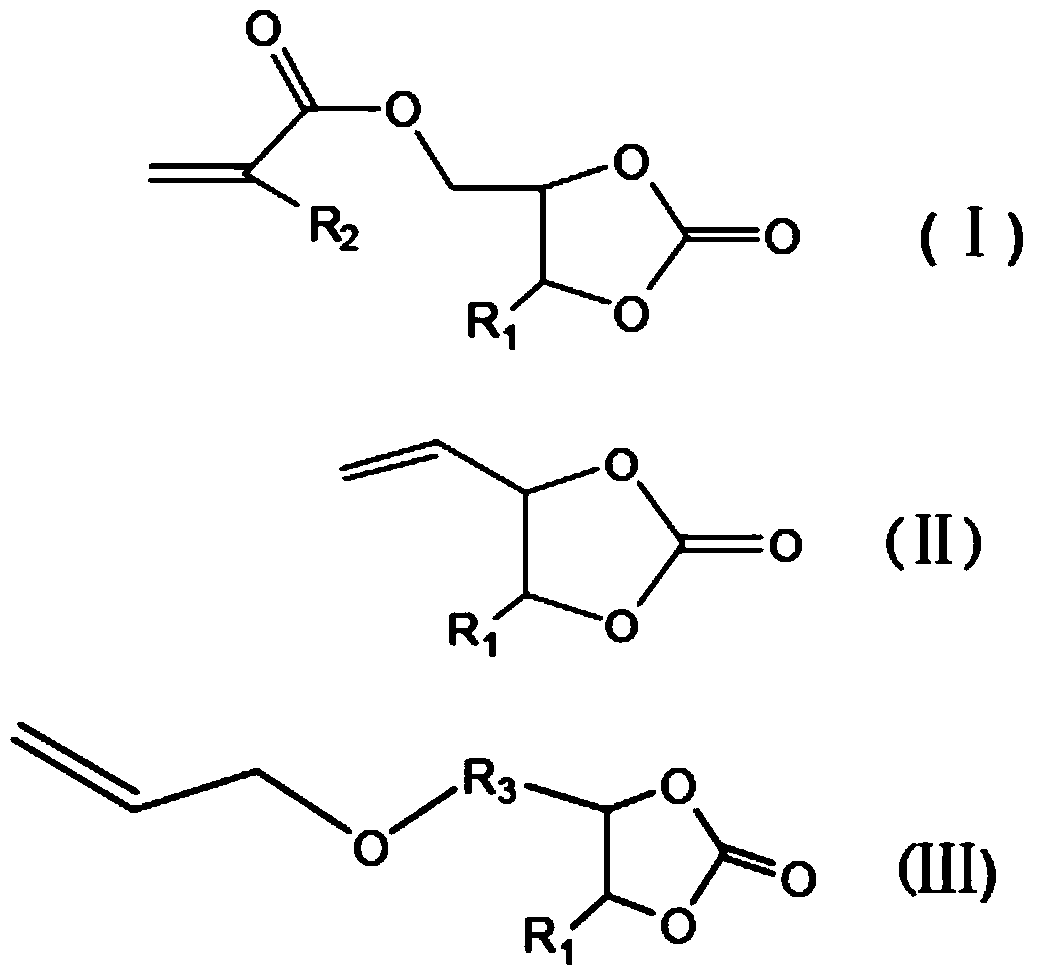
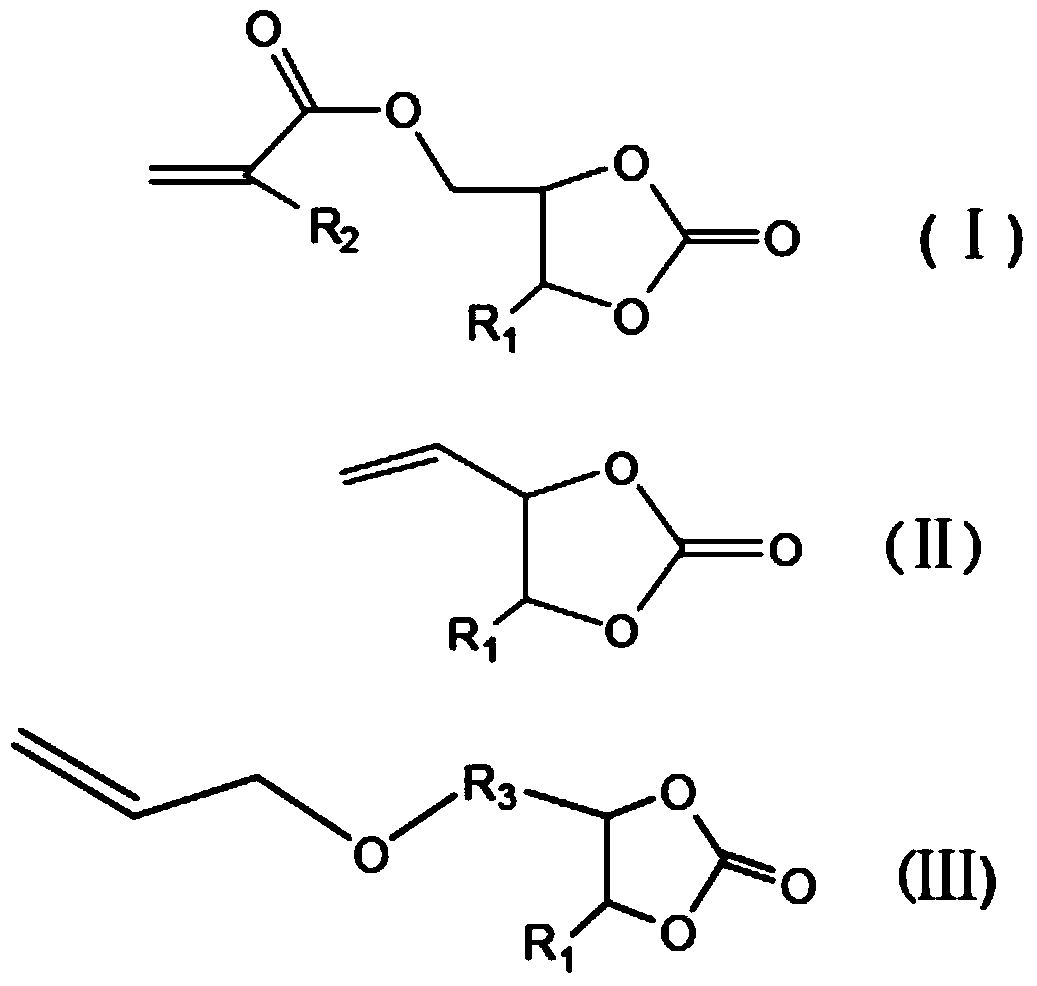
[0047]In this step, an unsaturated prepolymer containing a urethane bond is obtained by reacting a cyclic carbonate containing an unsaturated double bond with a primary amine, wherein the cyclic carbonate containing an unsaturated double bond can be (methyl) Acrylic acid cyclocarbonat...
Embodiment 1
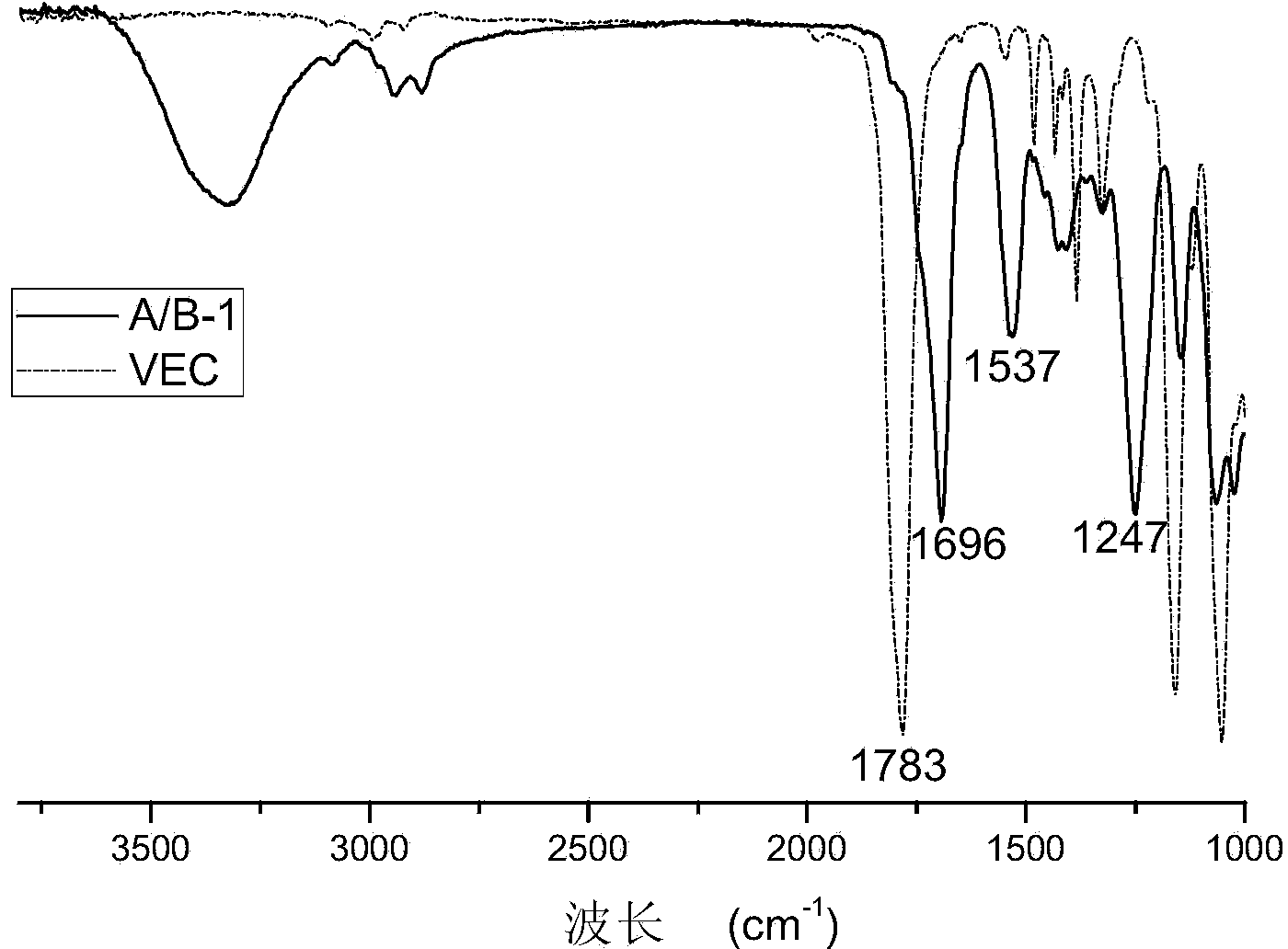
[0089] Step 1): Preparation of Prepolymer A / B-1
[0090] In a four-necked flask equipped with a thermometer, a stirrer, a condenser tube, and a nitrogen conduit, add 119.9 grams of vinyl cyclocarbonate (VEC), 61 grams of monoethanolamine, 0.5 gram of methylimidazole, methylhydroquinone (inhibitor) agent) 0.25 g, in N 2 Under gas protection, react at 120°C for 2-3h, raise the temperature to 150°C for 2-3h, then rise to 160°C for amine value ≤ 5mg KOH / g, cool down and discharge.
[0091] Step 2): Preparation of unsaturated polyester UPE-1
[0092] In a four-neck flask equipped with a thermometer, a stirrer, a water-splitting condenser, and a nitrogen conduit, all the raw materials in Table 1 below except xylene were put in order.
[0093] Table 1
[0094]
[0095] The reaction was carried out at 160-170°C for 2 hours, and the water generated by the reaction was blown with nitrogen, and then the temperature was raised by 10°C every 1 hour until 220°C, and 11.9 g of xylene w...
Embodiment 2
[0105] Step 1): Preparation of Prepolymer A / B-2
[0106] Reaction unit is the same as embodiment 1, adds methacrylic acid cyclocarbonate 195.3 grams, n-butylamine 73.1 grams, the methylimidazole of 0.38 grams, methylhydroquinone (polymerization inhibitor) 0.2 grams, in N 2 Under gas protection, react at 80°C for 2-3h, raise the temperature to 120°C for 2-3h, then raise the temperature to 150°C to react until the amine value is ≤5mg KOH / g, cool down and discharge.
[0107] Step 2): Preparation of unsaturated polyester UPE-2
[0108] The device and process are the same as in Example 1, and all the raw materials except xylene in the following Table 2 are put in successively, and the terminal acid value AV<3mg KOH / g.
[0109]
[0110] The number average molecular weight of the obtained resin is about 4300, the glass transition temperature is about -25°C, the theoretical hydroxyl value is 112 mg KOH / g, and the maleic anhydride content is 3.7%.
[0111] Step 3): Preparation of ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Acid value | aaaaa | aaaaa |
| Glass transition temperature | aaaaa | aaaaa |
| Theoretical hydroxyl value | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com