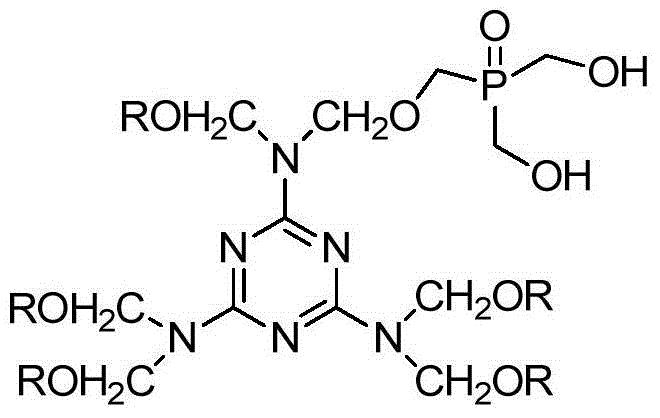
Phosphorus-nitrogen synergistic flame-retardant polyalcohol and preparation method thereof
A synergistic flame retardant and polyol technology, applied in chemical instruments and methods, compounds of Group 5/15 elements of the periodic table, organic chemistry, etc. Inexpensive, practical and easy to operate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] (1) Preparation of trihydroxymethylphosphine oxide (THPO)
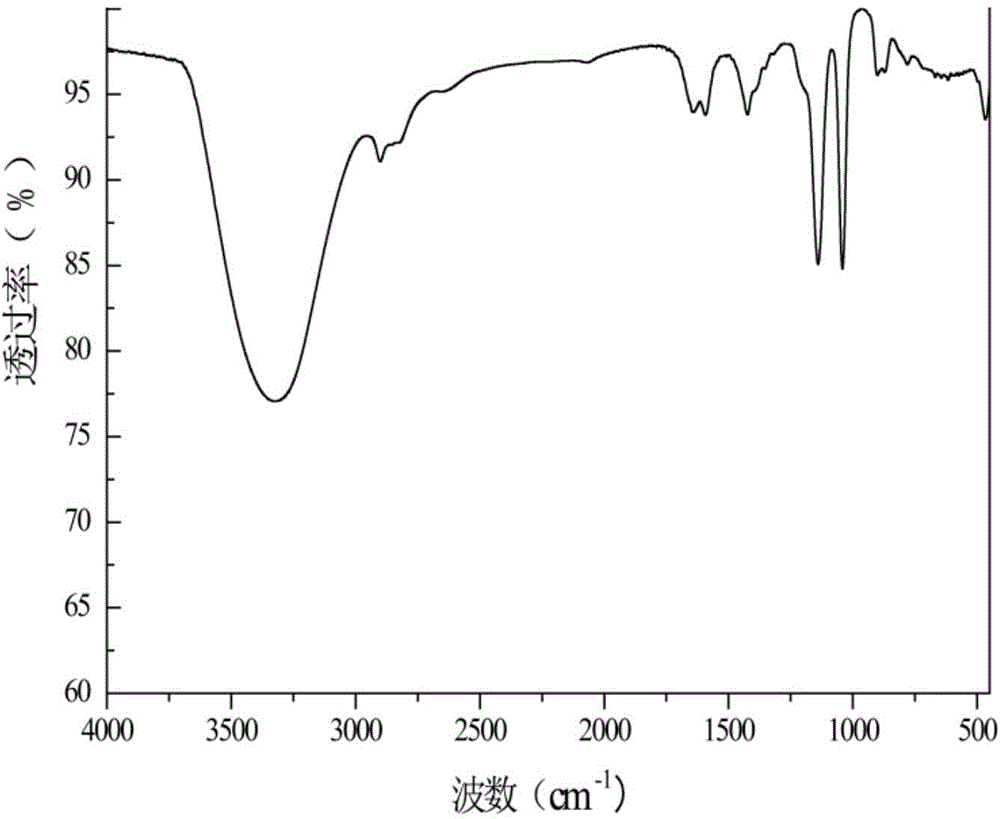
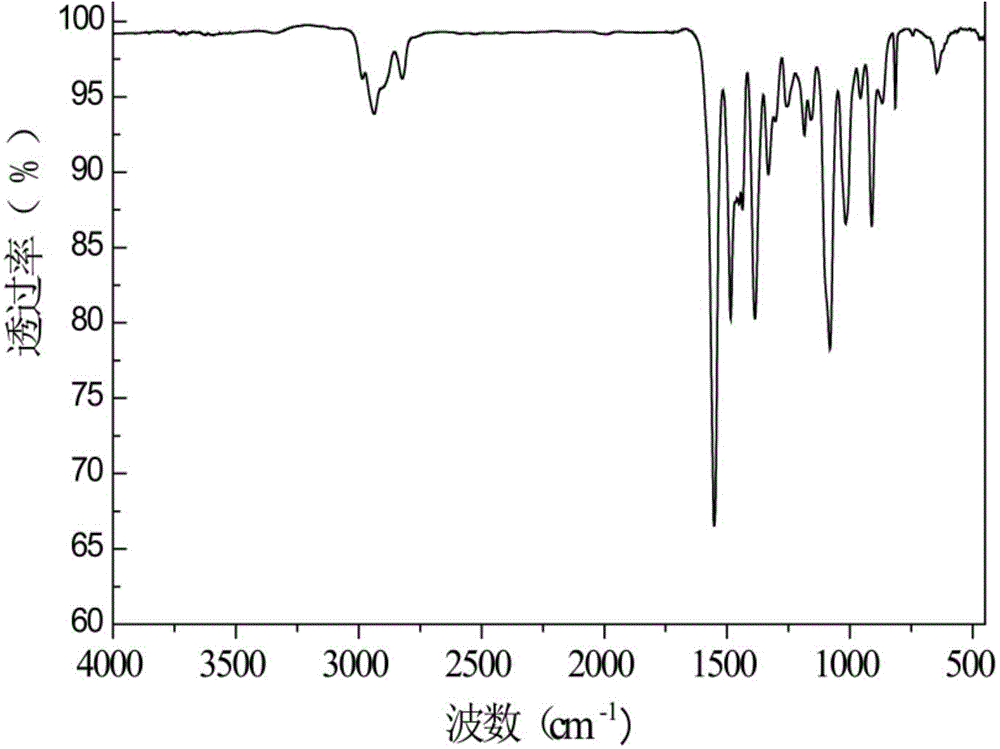
[0032] In a 1L three-necked flask equipped with a stirrer, a constant pressure dropping funnel, and a condenser, add 40.0 g (1 mol) of NaOH and 400 mL of distilled water, and stir to completely dissolve the alkali. After it was cooled to room temperature, 190.1 g (1 mol) of tetrakishydroxymethylphosphonium chloride solution was added dropwise through a constant pressure funnel. Dropping time 1h, 50 ℃ reaction 5h. After the reaction, slowly add HCl solution dropwise to the solution, after adjusting the pH to neutral, add 19mL HCl solution 2 o 2 After reacting for 2 hours, use a rotary evaporator to evaporate most of the water, then pour it into a 1L beaker containing 400mL of ethanol, and let it stand overnight until the salt is fully separated, then filter it with suction. The solvent is spin-dried to obtain the product THPO. And carried out infrared characterization, such as figure 1 shown.
[0033] (2) ...
Embodiment 2
[0036] (1) Preparation of trihydroxymethylphosphine oxide
[0037] Add 85.5g (0.5mol) Ba(OH) 2 , and add 300mL of distilled water, stir to dissolve the base completely. After it was cooled to room temperature, 190.1 g (1 mol) of tetrakishydroxymethylphosphonium chloride solution was added dropwise through a constant pressure funnel. Dropping time 1h, 70 ℃ reaction 4h. After the reaction was completed, HCl solution was slowly added dropwise to the solution to adjust the pH to neutral. Add 19mLH 2 o 2 After reacting for 2 hours, use a rotary evaporator to evaporate most of the water, then pour it into a 1L beaker containing 300mL of ethanol, and let it stand overnight until the salt is fully separated, then filter it with suction. The solvent is spin-dried to obtain the product.
[0038] (2) Preparation of Phosphorus Nitrogen Synergistic Flame Retardant Polyols
[0039] Experimental procedure 2 is the same as embodiment 1.
Embodiment 3
[0041] (1) Preparation of trihydroxymethylphosphine oxide
[0042]In a 1L three-necked flask equipped with a stirrer, a constant pressure dropping funnel, and a condenser, add 40.0 g (1 mol) of NaOH and 400 mL of distilled water, and stir to completely dissolve the alkali. After it was cooled to room temperature, 190.1 g (1 mol) of tetrakishydroxymethylphosphonium chloride solution was added dropwise through a constant pressure funnel. Dropping time 1h, 60 ℃ reaction 5h. After the reaction was completed, HCl solution was slowly added dropwise to the solution to adjust the pH to neutral. Add 19mLH 2 o 2 After reacting for 2 hours, use a rotary evaporator to evaporate most of the water, then pour it into a 1L beaker containing 400mL of ethanol, and let it stand overnight until the salt is fully separated, then filter it with suction. The solvent was spin-dried to obtain the product.
[0043] (2) Preparation of Phosphorus Nitrogen Synergistic Flame Retardant Polyols
[0044...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com