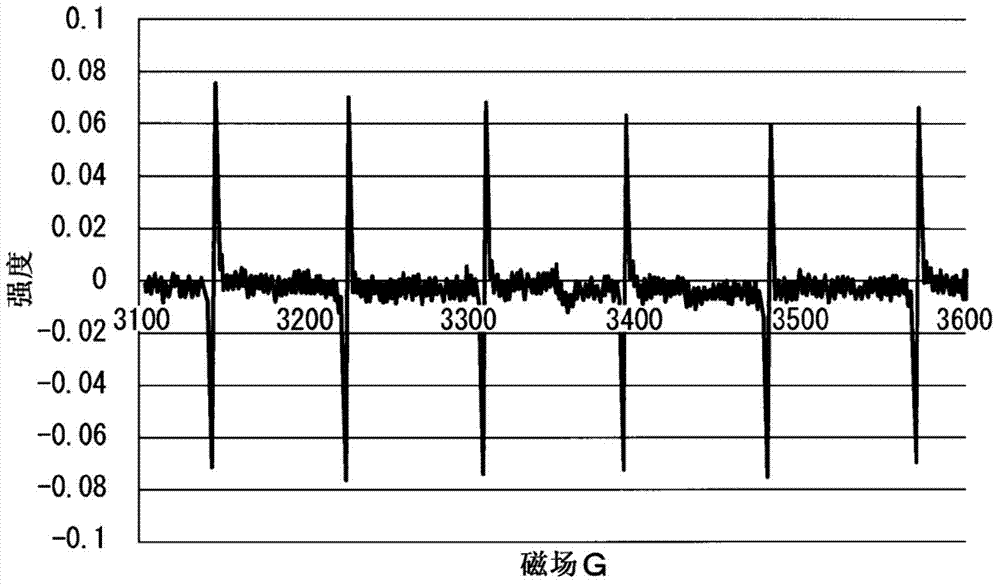
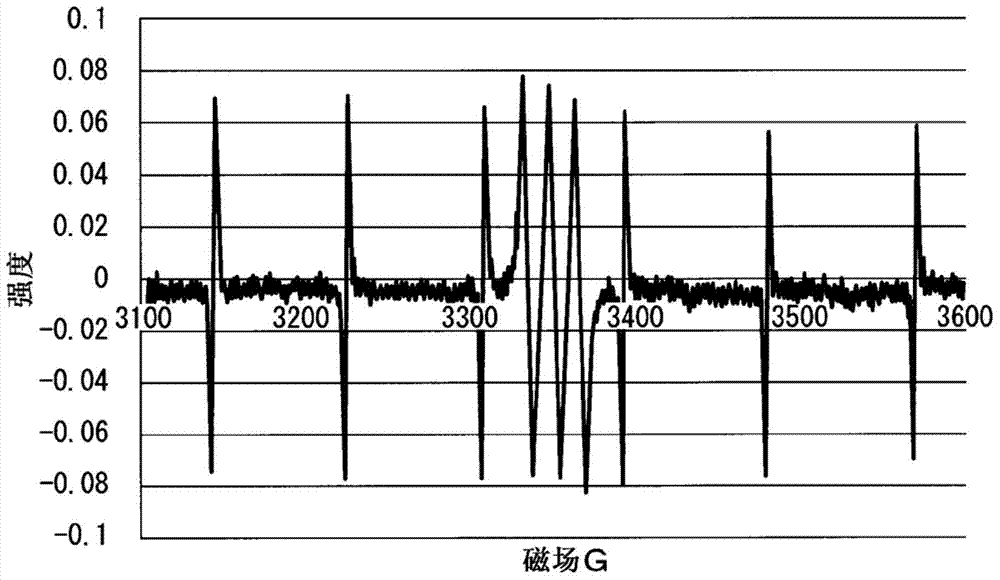
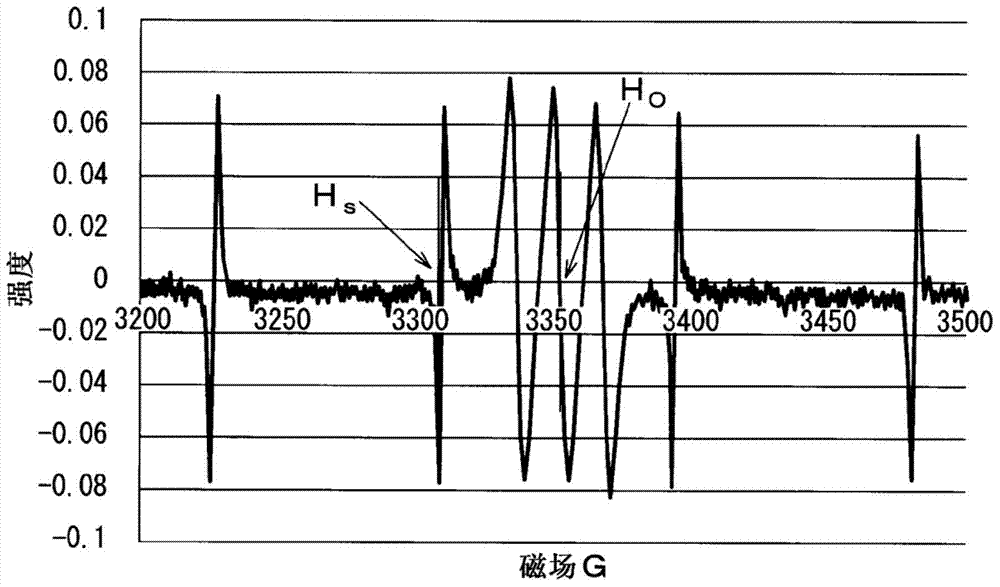
Polysiloxane composition having radical-crosslinkable group
A cross-linking group, polysiloxane technology, which is applied in the photoengraving process of the pattern surface, the photosensitive material for opto-mechanical equipment, optics, etc., can solve the problem of inability to complete thermal imidization, curing film Reduced physical properties and other problems, to achieve the effect of high transparency and excellent resistance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0217] Examples of the compound (a) used in the production of carboxyl group-containing vinyl polymers include (meth)acrylic acid, carboxyethyl (meth)acrylate, carboxypentyl (meth)acrylate, 2- (Meth)acryloyloxyethylsuccinic acid, 2-(meth)acryloyloxyethylhexahydrophthalic acid, 2-(meth)acryloyloxyethylphthalic acid, rich Maleic acid, cinnamic acid, crotonic acid, itaconic acid, and maleic acid half ester, etc. These may be used alone or in combination of two or more.
[0218] As the aforementioned compound (b) used in the preparation of the carboxyl group-containing vinyl polymer, for example, methyl (meth)acrylate, ethyl (meth)acrylate, n-propyl (meth)acrylate, ( Lauryl methacrylate, cyclohexyl (meth)acrylate, n-butyl (meth)acrylate, 2-ethylhexyl (meth)acrylate, 2-hydroxyethyl (meth)acrylate, (meth)acrylate base) 2-hydroxypropyl acrylate, 4-hydroxybutyl (meth)acrylate, caprolactone (meth)acrylate, nonylphenoxy polypropylene glycol (meth)acrylate, polyethylene glycol mono(met...
Embodiment 1~14、 Embodiment 16~27
[0321] (Regarding Examples 1 to 14, Examples 16 to 27, and Comparative Examples 1 to 5)
[0322] After the exposure, the alkaline developer (Developer, 2.38% aqueous solution of tetramethylammonium hydroxide) was used at 23°C for 30 seconds x 2 times, followed by rinsing with pure water to remove the residue of the coating film. unexposed part. Next, the above-mentioned patterned substrate was baked at 220° C. for 30 minutes in an oven replaced by nitrogen to cure it.
Embodiment 15
[0324] After the exposure, image development was performed using PGMEA (propylene glycol monomethyl ether acetate) at 23° C. for 30 seconds×2 times, and the unexposed portion of the coating film was removed. Next, the above-mentioned patterned substrate was baked at 220° C. for 30 minutes in an oven replaced by nitrogen to cure it.
[0325] The exposure amount of the patterns obtained by observing the patterns obtained in Examples 1-27 and Comparative Examples 1-5 with an optical microscope was 400 mJ / cm 2 The part which was shielded from light in a square of 25 μm×25 μm was evaluated according to the following criteria.
[0326] 〇: There is no residue, opening, and pattern floating or peeling is not observed
[0327] △: Opened but partly remained, no pattern floating or peeling was observed
[0328] ×: There is residue and no opening, or the pattern floats or peels off
[0329] 2. Evaluation of resolution range
[0330] The pattern obtained above was observed with an opti...
PUM
| Property | Measurement | Unit |
|---|---|---|
| acid value | aaaaa | aaaaa |
| acid value | aaaaa | aaaaa |
| acid value | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com