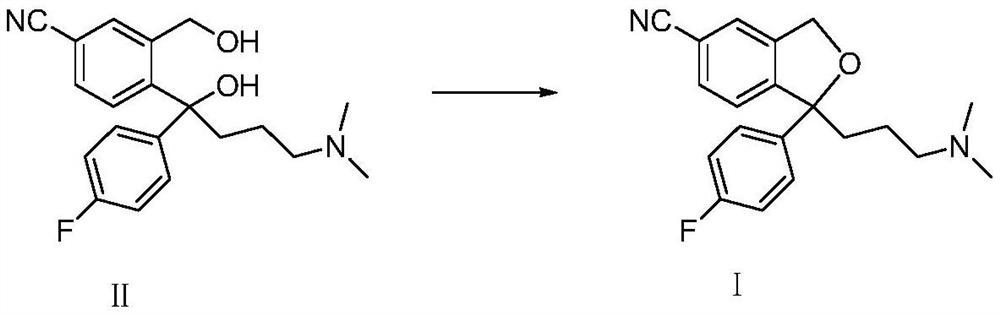
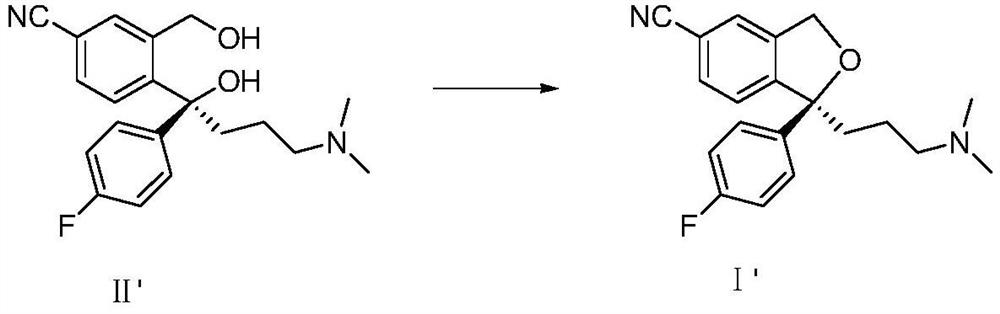
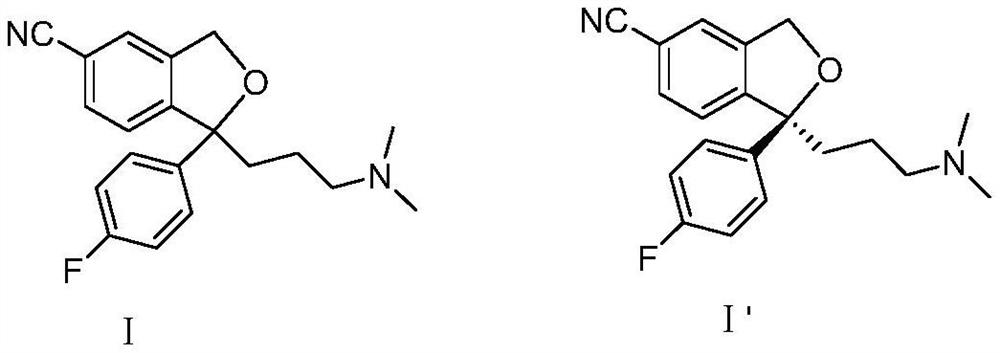
A kind of method for preparing citalopram and s-citalopram
A technology of organic solvent and arylsulfonyl chloride, applied in the field of preparing citalopram and S-citalopram, can solve the problems of low solvent recovery rate, large environmental pollution and high production cost, and achieve simple post-processing and high yield , the effect of increasing productivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] Add 34.2g of diol compound (II) and 100ml of methyl isobutyl ketone into a four-neck flask, stir to dissolve at room temperature, add 12g of NaOH, add 50ml of water, cool to 3°C, and slowly add 100ml of methyl alcohol A solution in which 27 g of p-toluenesulfonyl chloride was dissolved in isobutyl ketone was heated up to room temperature after the reaction was completed at 3°C. The layers were allowed to stand, and the aqueous layer was extracted once with 70ml of methyl isobutyl ketone; the combined organic layers were washed twice with 100ml of water each time. The yield of citalopram obtained by concentrating the organic layer was 83%, and the HPLC analysis result showed that the product had a purity of 99.6%.
Embodiment 2
[0027] Add 34.2g of S-diol compound (II') into the four-neck flask, add 100ml of methyl isobutyl ketone, stir at room temperature to dissolve, add 12g of NaOH, add 50ml of water, cool to 3°C, and then slowly dropwise add A solution of 27 g of p-toluenesulfonyl chloride was dissolved in 100 ml of methyl isobutyl ketone, and after the reaction was completed at 3° C., the temperature was raised to room temperature. The layers were allowed to stand, and the aqueous layer was extracted once with 70ml of methyl isobutyl ketone; the combined organic layers were washed twice with 100ml of water each time. The yield of S-citalopram (I') obtained by concentrating the organic layer was 85%, and the HPLC analysis results showed that the product had a purity of 99.8%.
Embodiment 3
[0029] Add 34.2g of diol compound (II) and 100ml of methyl isobutyl ketone into a four-neck flask, stir to dissolve at room temperature, add 12g of NaOH, add 50ml of water, cool to 3°C, and slowly add 100ml of A solution in which 16 g of methanesulfonyl chloride was dissolved in methyl isobutyl ketone was heated up to room temperature after the reaction was completed at 3°C. The layers were allowed to stand, and the aqueous layer was extracted once with 70ml of methyl isobutyl ketone; the combined organic layers were washed twice with 100ml of water each time. The yield of citalopram obtained by concentrating the organic layer was 80%, and HPLC analysis showed that the product had a purity of 99.5%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com