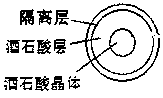
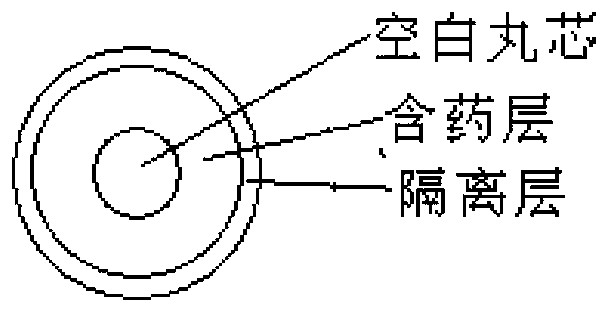
Oral double pellet pharmaceutical composition of dabigatran etexilate or its salt
A technology of dabigatran etexilate and double pellets, which is applied in the direction of drug combination, pill delivery, pharmaceutical formulation, etc., and can solve the problems affecting the roundness of tartaric acid pellet core, the surface irregularity of tartaric acid core core, and the stability of active substances sexual issues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0055] Prescription and process:
[0056] 1. Preparation of pills containing:
[0057] 1) List of prescriptions containing pills:
[0058]
[0059]
[0060] 2) The preparation method of containing pills:
[0061] a) Pill core with pills: Add the HPC-EF in the proportion of the prescribed amount into the isopropanol solution, stir to dissolve, add the other components in the prescribed amount and stir to form a uniform suspension, and set aside. Take the sucrose core in the proportion of the prescription and put it into the miniGlatt fluidized bed coating machine, and gradually spray the coating solution on the outside of the sucrose core under the following parameter conditions until a suitable pellet is formed.
[0062] Coating parameters: fluidization pressure 0.15-0.30 bar, atomization pressure 1.20-1.50 bar, liquid feeding speed 3.0-5.0, pellet temperature 20-25 °C.
[0063] b) Isolation gown containing pills: Disperse the prescribed amount of Opadry in purified w...
experiment example
[0077] The inventors conducted in vitro dissolution behavior investigations and in vivo biological studies on capsules of different combinations of the pellets of the examples in the present invention.
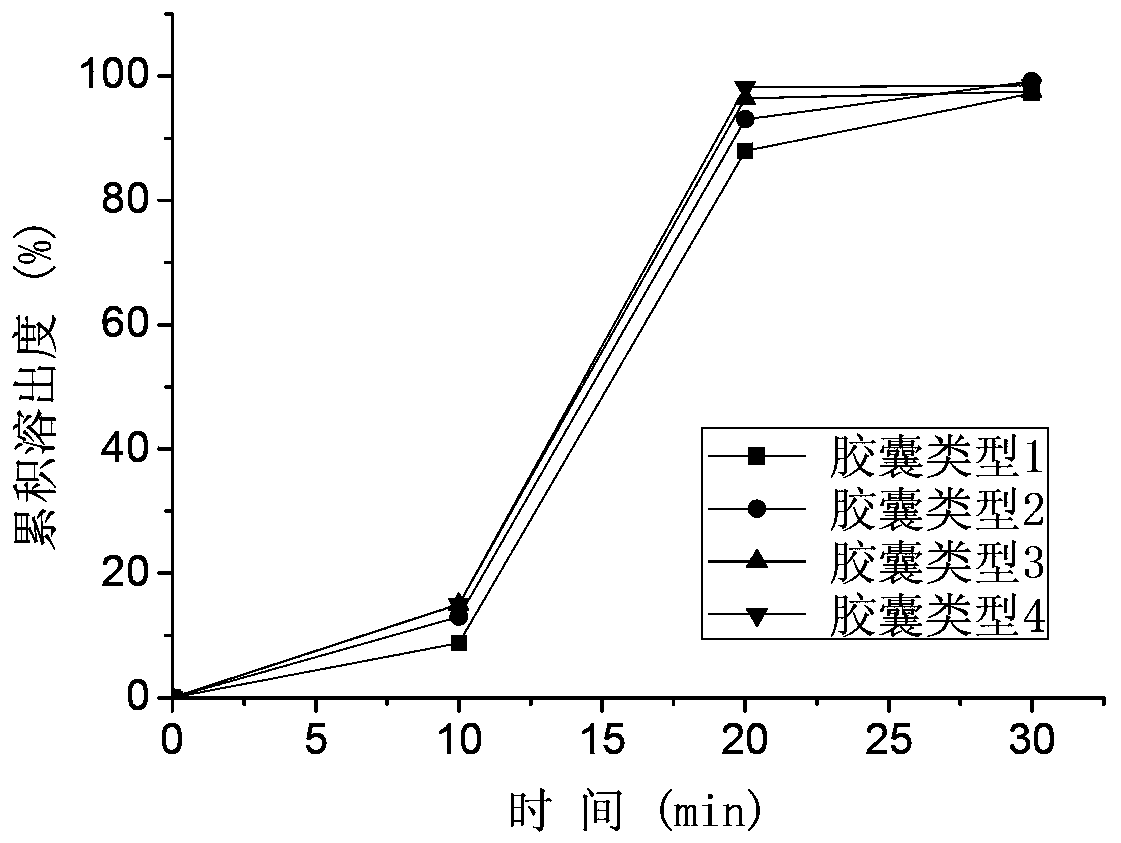
[0078] In vitro study: 900ml of 0.01M HCl, purified water, and pH 4.5 acetate buffer salt were used as dissolution media respectively, and the basket method was performed at 100 rpm to investigate the in vitro dissolution rate of the pellets prepared in the examples. Among them, 0.01M HCl and purified water are the main investigation media, and it is stipulated that the cumulative dissolution rate of the drug in the pellets in 0.01M HCl should not be lower than 85% in 30 minutes, the cumulative dissolution rate in purified water for 30 minutes should not be lower than 70%, and the cumulative dissolution rate in 60 minutes should not be less than 85%. less than 85%. Dissolution curve see attached Figure 3-7 .
[0079] The results of in vitro study showed that different formu...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com