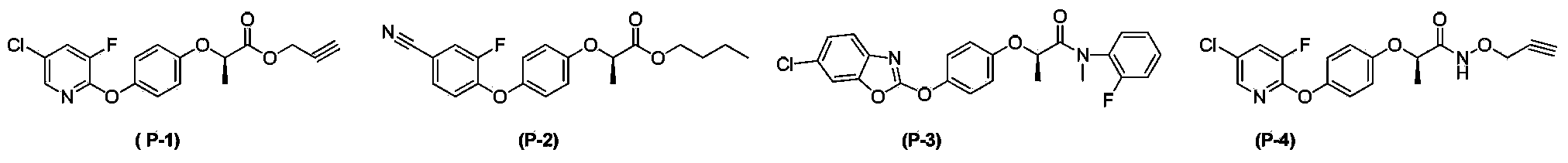
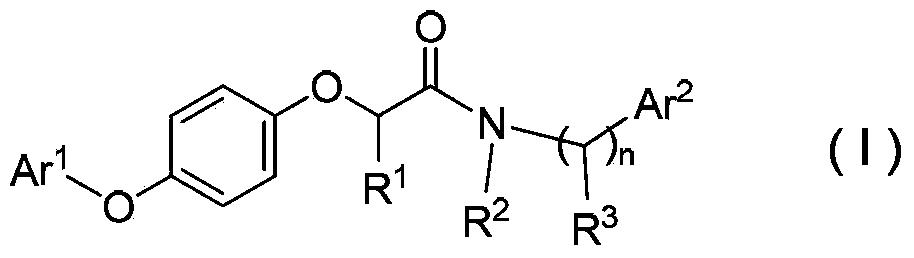
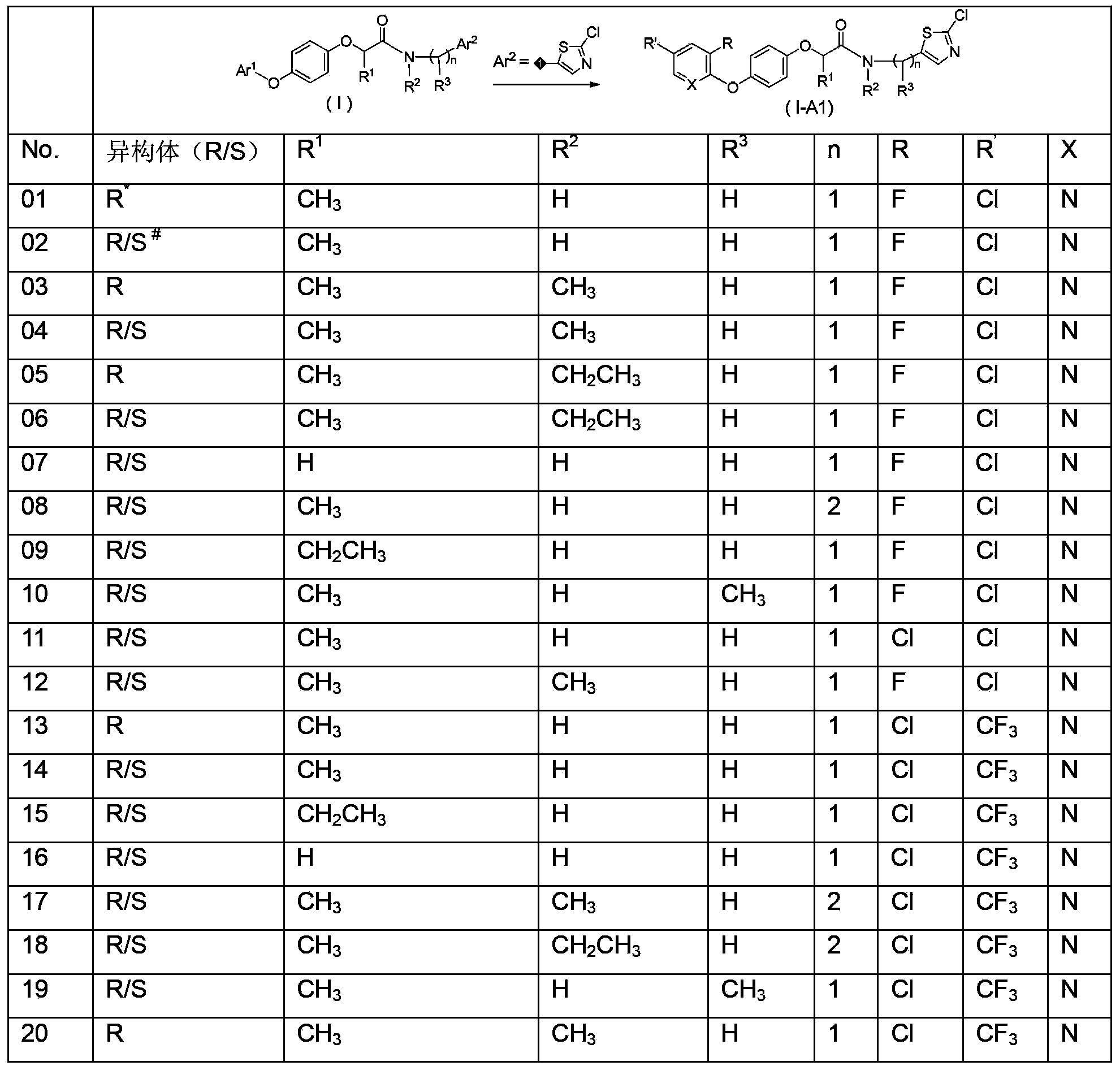
N-(arylalkyl)aryloxy phenoxy carboxylic acid amide compounds, and preparation method and application thereof
A technology of aryloxyphenoxycarboxylic acid amide and aryl alkyl, which is applied in the field of N-aryloxyphenoxycarboxylic acid amide compounds, and can solve the problems of unsafe rice, low activity, and inability to control weeds in paddy fields, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0108] This example illustrates the preparation of compound 01 in Table 1.
[0109]
[0110] (R)-2-(4-((5-chloro-3-fluoropyridin-2-yl)oxy)phenoxy)propionic acid was added in a 100mL three-necked flask equipped with a magnetic stirrer, thermometer and condenser N,N-Dimethylformamide (DMF) (40mL), (R) 2-(4-hydroxyphenoxy)propionic acid (0.02mol), after stirring and dissolving, add potassium carbonate (0.04mol) in batches, Raise the temperature to 60-80°C and stir for 0.5-1.0 hr. 2,3-Difluoro-5-chloropyridine (0.02 mol) was added dropwise, and the stirring reaction was continued for 6-8 hr. The reactant was cooled to room temperature, poured into ice water (250 mL), and dilute hydrochloric acid was slowly added to the mixture to adjust the pH to 4-5, filtered, washed with water, and dried to obtain 5.92 g of the title compound as a white solid.
[0111] Add N,N-dimethylformamide (40mL), phthalimide (0.05mol) and potassium hydroxide (0.05mol) into a 100mL three-necked flask o...
Embodiment 2
[0114] This example illustrates the preparation of compound 03 in Table 1.
[0115]
[0116] Add 2-chloro-5-chloromethylthiazole (0.05mol), CHCl 3 (50mL), after stirring evenly, add dropwise methylamine aqueous solution (25g, 25-30%) and sodium hydroxide aqueous solution (25ml, 10%), and add a small amount of phase transfer catalyst tetrabutylammonium bromide, and stir at 25-30°C React overnight. Adjust the pH to weakly alkaline with dilute hydrochloric acid, separate the layers, wash the organic phase twice with water, dry over anhydrous sodium sulfate, and extract 6.67 g of the title compound as a pale orange liquid.
[0117] (R)-N-methyl-N-(2-chlorothiazol-5-ylmethyl)-2-(4-((5-chloro-3-fluoropyridin-2-yl)oxy)phenoxy ) Propionamide (compound 03 in Table 1) was added toluene (40mL) in 100mL one-mouth bottle, (R)-2-(4-((3-fluoro-5-chloropyridin-2-yl)oxy)phenoxy ) propionic acid (3.3 mmol) and thionyl chloride (10 mmol). After the reactant was refluxed for 3-5hr, the tol...
Embodiment 3
[0118] Example 3 This example illustrates the preparation of compound 42 in Table 1.
[0119]
[0120] (R)-2-(4-((3-Chloro-5-trifluoromethylpyridin-2-yl)oxy)phenoxy)propionic acid was prepared in a 100mL three-port vessel equipped with a magnetic stirrer, a thermometer and a condenser Add N,N-dimethylformamide (DMF) (40mL), (R)-2-(4-hydroxyphenoxy)propionic acid ([α] 589 20℃ =+59.5° (acetone)) (0.02mol), stir and dissolve, add potassium carbonate (0.04mol) in batches, raise the temperature to 60-80°C and stir for 1.0-1.5hr. 2,3-dichloro-5-trifluoromethylpyridine (0.02 mol) was added dropwise, and the stirring reaction was continued for 8-10 hrs. The reactant was cooled to room temperature, poured into ice water (250 mL), then slowly added dilute hydrochloric acid to the mixture, adjusted to pH=4~5, filtered, washed with water, and dried to obtain 6.56 g of the title compound as a white solid, [α] 589 20℃ =+37.9° (acetone).
[0121] Add N,N-dimethylformamide (DMF) (40mL...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com