Preparation method, detecting method and application of moxifloxacin hydrochloride impurity
A technology for moxifloxacin hydrochloride and impurities, which is applied in the field of medicinal chemistry, can solve problems such as operation danger, and achieve the effects of good control and simple preparation method.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
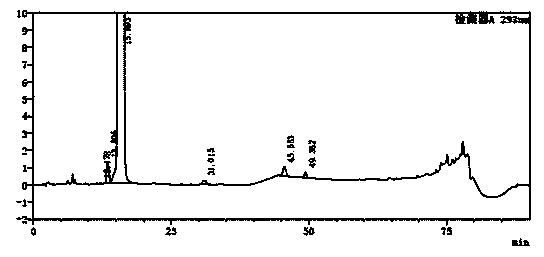
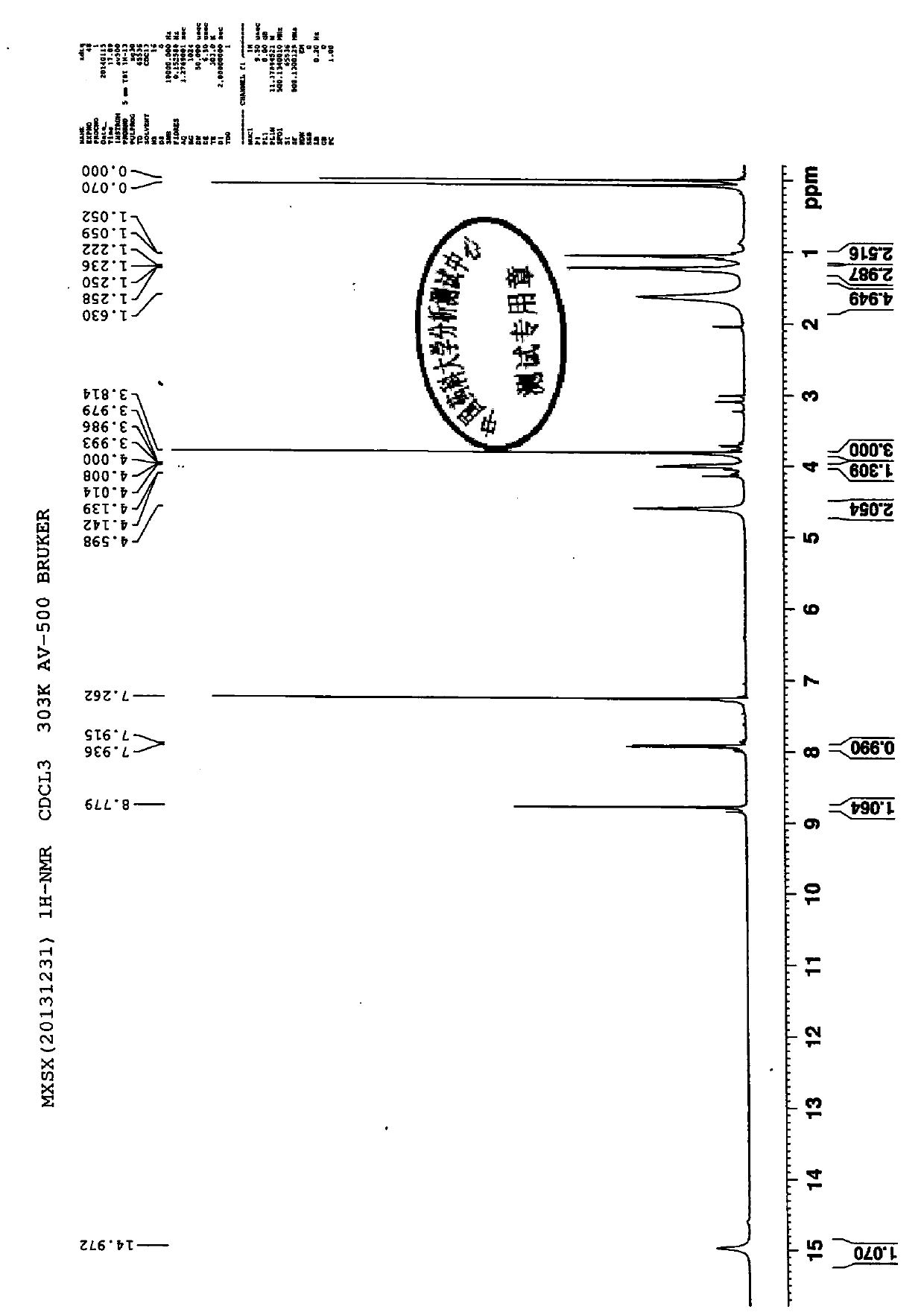
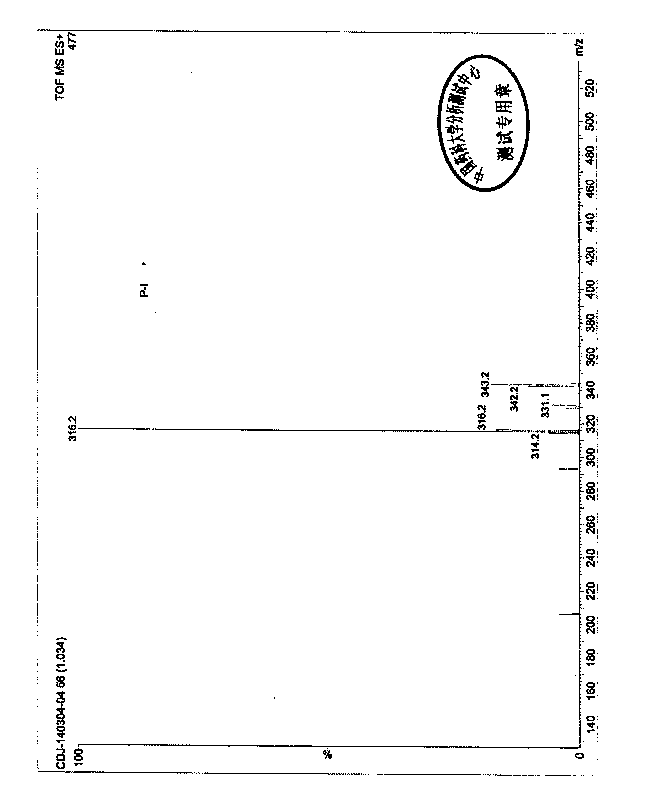
Image
Examples
Embodiment 1
[0052] 1-Cyclopropyl-7-(2,4-dimethoxybenzylamino)-6-fluoro-8-methoxy-1,4-dihydro-4-oxo-3-quinolinecarboxylic acid ethyl ester preparation of
[0053] Add 100g of ethyl 1-cyclopropyl-6,7-difluoro-8-methoxy-1,4-dihydro-4-oxo-3-quinolinecarboxylate into the three-necked flask, and then add 600mL of DMF to it: DMSO=1:1 mixed solvent, stir, add 54mL of 2,4-dimethoxybenzylamine, raise the temperature to 120°C for 6h, cool down, pour the reaction solution into 1mol / L dilute hydrochloric acid, stir, add 500mLEA to extract , the organic layer was separated, and the aqueous phase was extracted with EA 300mL×2. The organic layers were combined, dried, filtered and evaporated to dryness to obtain 92.3g of a yellow product, which was directly used in the next step without purification.
Embodiment 2
[0055] 1-Cyclopropyl-7-(2,4-dimethoxybenzylamino)-6-fluoro-8-methoxy-1,4-dihydro-4-oxo-3-quinolinecarboxylic acid ethyl ester preparation of
[0056]Add 100g of ethyl 1-cyclopropyl-6,7-difluoro-8-methoxy-1,4-dihydro-4-oxo-3-quinolinecarboxylate into the three-necked flask, then add 600mL of acetone to it, Stir, add 45mL of p-methoxybenzylamine, raise the temperature to 120°C for 6h, cool down, pour the reaction solution into 1mol / L dilute hydrochloric acid, stir, add 500mLEA for extraction, separate the organic layer, and add 300mL×2 EA to the water phase Extract, combine the organic layers, dry, filter and evaporate to dryness to obtain 92.3 g of yellow product, which is directly used in the next step without purification.
Embodiment 3
[0058] 1-Cyclopropyl-7-(2,4-dimethoxybenzylamino)-6-fluoro-8-methoxy-1,4-dihydro-4-oxo-3-quinolinecarboxylic acid ethyl ester preparation of
[0059] Add 100g of ethyl 1-cyclopropyl-6,7-difluoro-8-methoxy-1,4-dihydro-4-oxo-3-quinolinecarboxylate into the three-necked flask, and then add 600mL of DMF to it: DMSO=1:1 mixed solvent, stir, add 62.2mL of 2,3,4-trimethoxybenzylamine, raise the temperature to 120°C for 6h, cool down, pour the reaction solution into 1mol / L dilute hydrochloric acid, stir, add Extract with 500mL EA, separate the organic layer, extract the aqueous phase with 300mL×2 EA, combine the organic layers, dry, filter and evaporate to dryness to obtain 92.3g of a yellow product, which is directly used in the next step without purification.
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com