HRP5 analogues and preparation method thereof
An amino acid and sequence technology, applied in the field of medicine, can solve problems such as toxicity and high killing activity of pathogens
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0067] Embodiment 1: Preparation and purification of AMP-1
[0068] Sequence: Lys-Arg-Leu-Phe-Lys-Lys-Leu-Leu-Lys-Tyr-Leu-Arg-Lys-Phe-NH2 (SEQ ID NO: 3)
[0069] (1) Materials and reagents
[0070] Rink Amide MBHA resin, substitution value 0.41mmol / g.
[0071] The amino acids to be protected are Fmoc-L-Arg(Pbf)-OH, Fmoc-L-Leu-OH, Fmoc-L-Lys(Boc)-OH, Fmoc-L-Phe-OH and Fmoc-L-Tyr (tBu)-OH.
[0072] Reagents: HOBt, DIC, DMF, piperidine.
[0073] (2) Instrument
[0074] PSI300 peptide synthesizer, Waters600 semi-preparative high performance liquid chromatography, magnetic stirrer.
[0075] (3) Operation steps (take 0.25mmol as an example)
[0076] a. Solid-phase chemical synthesis of peptides
[0077] Weigh 0.61g of Rink Amide MBHA resin, put it in the reactor of the peptide synthesizer, add 10mL of DMF, soak for 2h, then add 15mL of 20% PIP / DMF solution, mix for 30min to remove the amino protecting agent, and wash the resin 7 times with DMF , then add 387.4mg Fmoc-L-Phe-O...
Embodiment 2
[0092] Example 2: Preparation and purification of HRP5 analogues in Table 4
[0093] (1) Materials and reagents
[0094] Rink Amide MBHA resin, substitution value 0.41mmol / g.
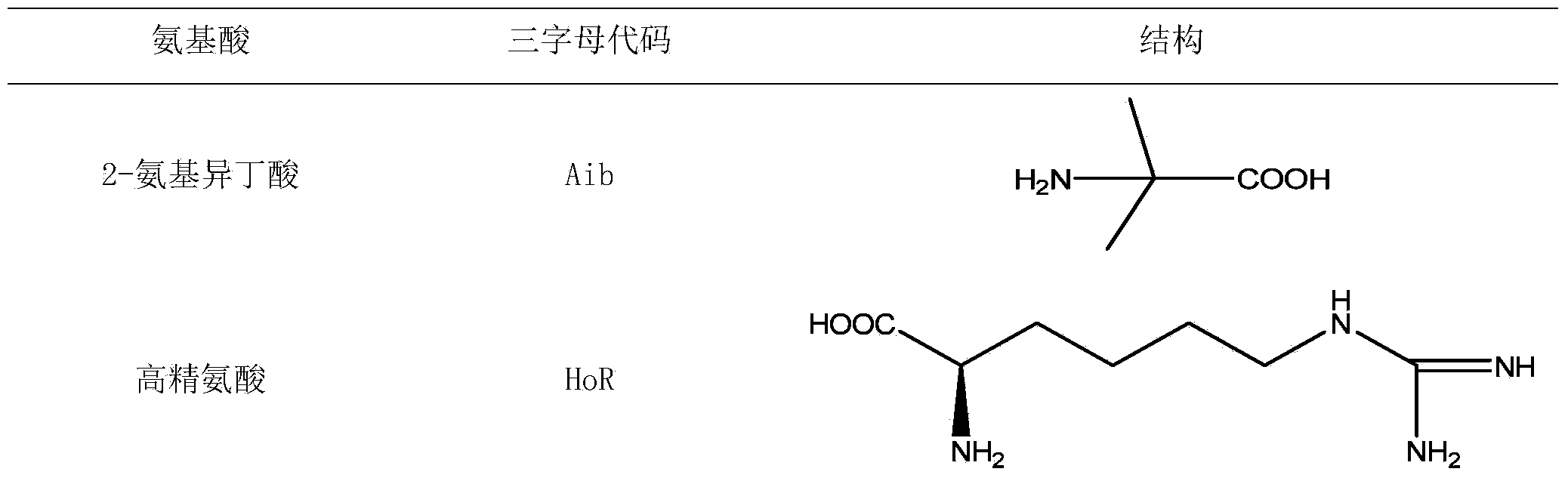
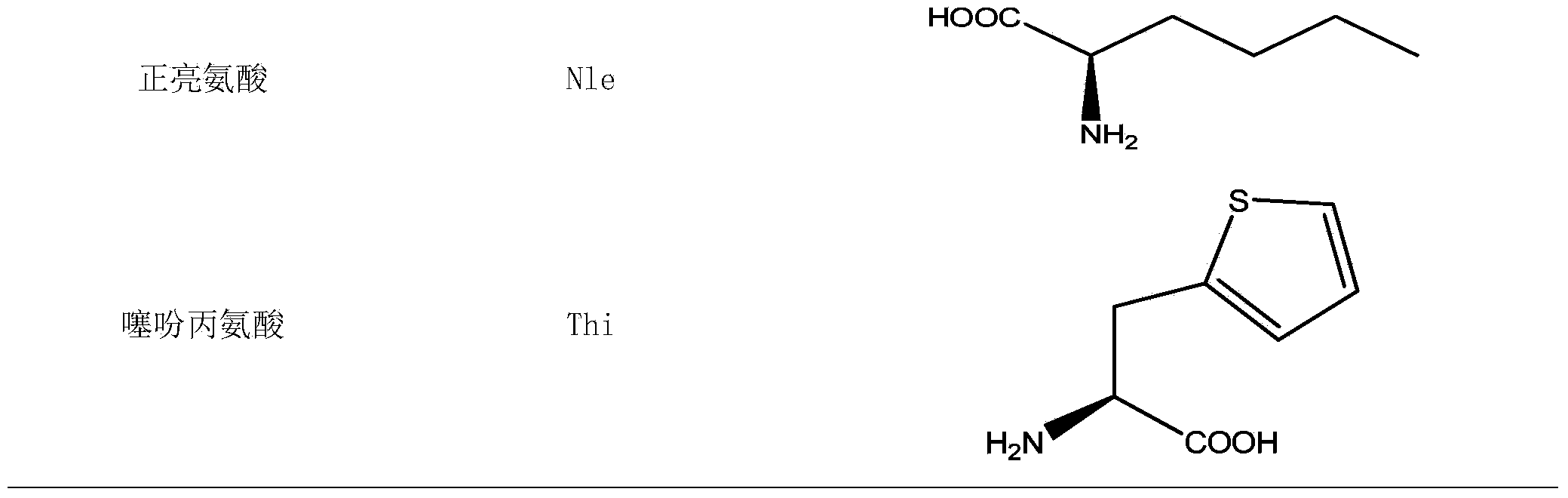
[0095] Desired protected amino acids Fmoc-L-Arg(Pbf)-OH, Fmoc-D-Arg(Pbf)-OH, Fmoc-L-Leu-OH, Fmoc-L-HoR(Pbf)-OH, Fmoc-L -Lys(Boc)-OH, Fmoc-D-Lys(Boc)-OH, Fmoc-L-Phe-OH, Fmoc-D-Phe-OH, Fmoc-L-Tyr(tBu)-OH, Fmoc-L- Ile-OH, Fmoc-L-Nle-OH, Fmoc-L-Thi-OH and Fmoc-L-Aib-OH.
[0096] Reagents: HOBt, DIC, DMF, piperidine.
[0097] (2) Instrument
[0098] PSI300 peptide synthesizer, Waters600 semi-preparative high performance liquid chromatography, magnetic stirrer.
[0099] (3) Operation steps (take 0.25mmol as an example)
[0100] The polypeptides in Table 4 were prepared and purified in a manner similar to the steps a-c in Example 1, and the fractions with a purity greater than 99% were collected and then concentrated to dryness at 50°C under reduced pressure. The ESI-MS measured values are shown in Ta...
Embodiment 3
[0103] Example 3: Preparation and purification of cyclic peptide AMP-13
[0104] sequence: (SEQ ID NO: 15)
[0105] (1) Materials and reagents
[0106] Rink Amide MBHA resin, substitution value 0.41mmol / g.
[0107] The amino acids to be protected are Fmoc-L-Arg(Pbf)-OH, Fmoc-L-Leu-OH, Fmoc-L-Lys(Boc)-OH, Fmoc-L-Phe-OH, Fmoc-L-Tyr (tBu)-OH and Fmoc-L-Cys(Trt)-OH.
[0108] Reagents: HOBt, DIC, DMF, piperidine, 30% H 2 o 2 .
[0109] (2) Instrument
[0110] PSI300 peptide synthesizer, Waters600 semi-preparative high performance liquid chromatography, magnetic stirrer.
[0111] (3) Operation steps
[0112] a. Prepared and purified by a method similar to the operation steps a-c in Example 1. Cys-NH 2 ), the fractions with a purity greater than 99% were collected, and then concentrated to dryness at 50°C under reduced pressure. As determined by ESI-MS, the molecular weight of the peptide is 2086.24, and the theoretical value is 2086.72.
[0113] b. Intramolecular disul...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com