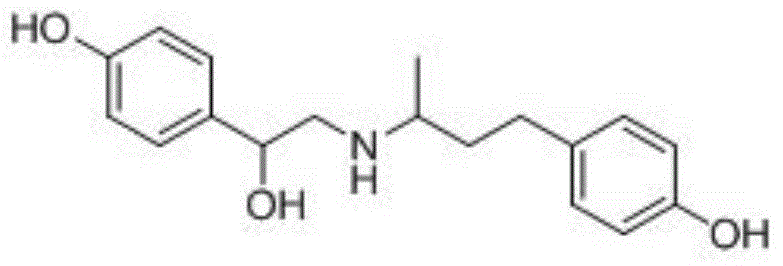
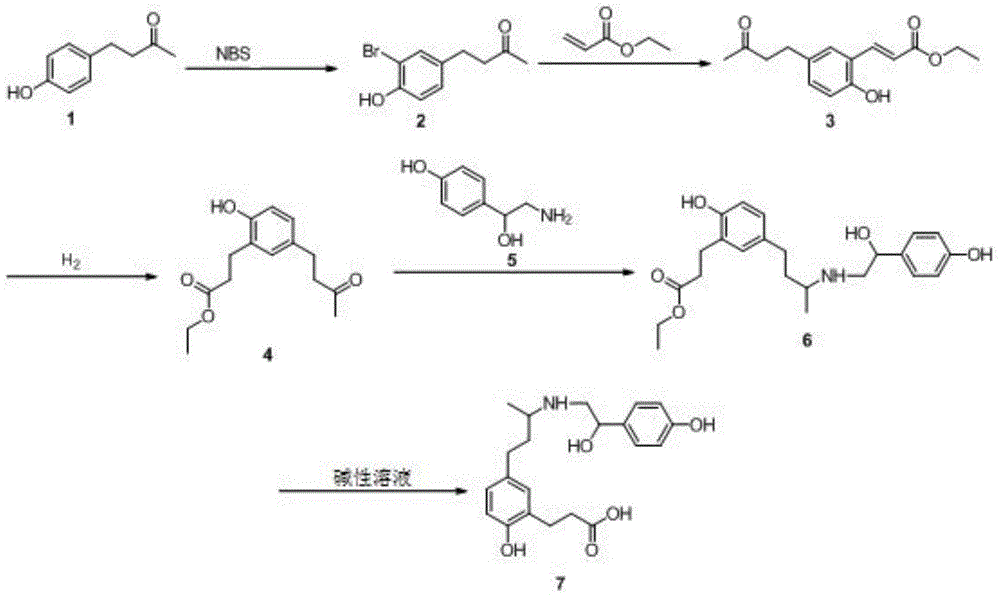
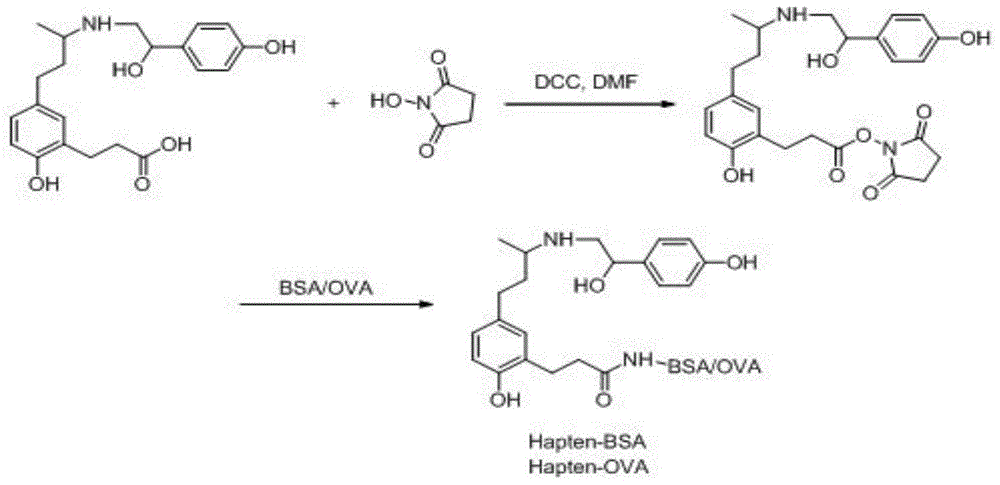
Ractopamine semiantigen, artificial antigen and monoclonal antibody, and preparation methods and applications thereof
A technology of ractopamine mono- and ractopamine, which is applied in the field of ractopamine hapten, monoclonal antibody and its preparation, and artificial antigen, can solve the problems of high production cost, low sensitivity of kit, low sensitivity of monoclonal antibody, etc., and achieve Improve sensitivity and potency, reduce cost, and achieve high specificity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0048] 1. Preparation of ractopamine hapten
[0049] The specific experimental steps are:
[0050] (1) Dissolve compound 1 (164mg, 1.0mmol, 1.0eq) in 4mL acetonitrile, add NBS (N-bromosuccinimide) (200mg, 1.1mmol, 1.1eq) under stirring, and stir at room temperature for reaction 3h. Add 25mL H after the reaction 2 O, EA (ethyl acetate) (3×10 mL) extraction, combined organic phases, dried over anhydrous sodium sulfate, filtered, concentrated under reduced pressure, dry column chromatography, DCM (dichloromethane) as eluent, atmospheric pressure After elution, 209 mg of yellow oily substance, intermediate 2, was obtained. Yield: 86%. 1H NMR (400MHz, CDCl 3 )δ7.28(d,J=1.5Hz,1H), 7.02(dd,J=8.3,1.6Hz,1H), 6.91(d,J=8.3Hz,1H), 5.62(s,1H), 2.79( d, J=6.9 Hz, 2H), 2.73 (d, J=7.0 Hz, 2H), 2.14 (s, 3H).
[0051] (2) Intermediate 2 (1.75g, 7.29mmol, 1.0eq) was added to a dry two-necked flask, and [1,1'-bis(diphenylphosphino)ferrocene]palladium dichloride (970mg, 1.46mmol, 0.2eq), swap N 2 R...
Embodiment 2
[0149] Preparation of ractopamine hapten
[0150] The specific experimental steps are:
[0151] (1) Dissolve compound 1 (164mg, 1.0mmol, 1.0eq) in 4mL acetonitrile, add NBS (N-bromosuccinimide) (240mg, 1.5mmol, 1.5eq) under stirring, and stir at room temperature for reaction 4h. Add 25mL H after the reaction 2 O, EA (ethyl acetate) (3×10 mL) extraction, combined organic phases, dried over anhydrous sodium sulfate, filtered, concentrated under reduced pressure, dry column chromatography, DCM (dichloromethane) as eluent, atmospheric pressure After elution, 345 mg of yellow oily substance, intermediate 2, was obtained. Yield: 94.6%. 1H NMR (400MHz, CDCl 3 )δ7.28(d,J=1.5Hz,1H), 7.02(dd,J=8.3,1.6Hz,1H), 6.91(d,J=8.3Hz,1H), 5.62(s,1H), 2.79( d, J=6.9 Hz, 2H), 2.73 (d, J=7.0 Hz, 2H), 2.14 (s, 3H).
[0152] (2) Intermediate 2 (0.88g, 3.6mmol, 1.0eq) was added to a dry two-necked flask, and [1,1'-bis(diphenylphosphino)ferrocene]palladium dichloride (485mg, 0.73mmol, 0.2eq), swap N 2 Rep...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com