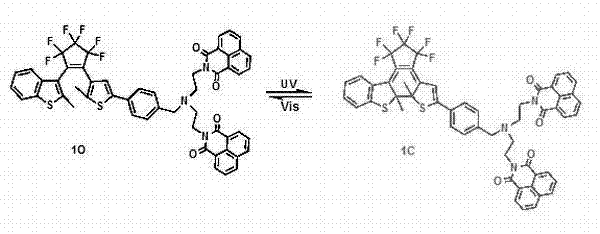
Photochromic bis(N-ethyl-1,8-naphthalimide)amine-benzothiophene hybrid type perfluorocyclopentene compound, and synthetic method and application thereof
A perfluorocyclopentene, photochromic technology, applied in chemical instruments and methods, color-changing fluorescent materials, luminescent materials, etc., to achieve the effect of broad application prospects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
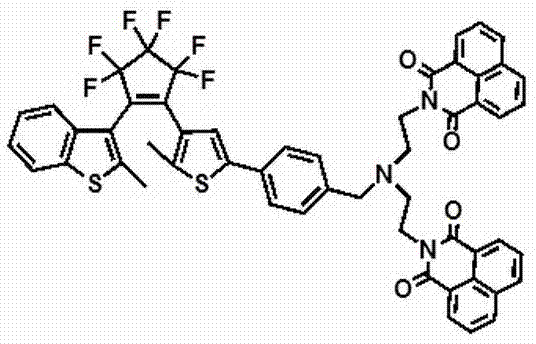
[0025] The name is: 1-(2-dimethyl-3-benzothienyl), {2-[2-methyl-5-(4-((bis(N-ethyl-1,8-naphthoylidene) Amine)amine)methyl)-phenyl)]-3-thienyl}perfluorocyclopentene, the structural formula is as follows:
[0026]
[0027] The synthetic scheme Scheme 1 of this novel diaryl perfluorocyclopentene photochromic compound is shown in:
[0028] Scheme1:
[0029] Its specific synthetic steps are as follows:
[0030] 1. 1-(2-Methylbenzothiophen-1-yl) perfluorocyclopentene (3):
[0031] Dissolve compound 2 (3.0g, 13.5mmol) in 60 ml THF, stir under nitrogen and -78°C, slowly inject 2.5 mol / L n-BuLi (5.9ml, 14.9mmol), and continue stirring at low temperature for half an hour . Inject perfluorocyclopentene (2 mL, 14.9 mmol) into the reaction flask, continue to stir the reaction mixture at low temperature for 1 h, then naturally rise to room temperature, add an appropriate amount of water to terminate the reaction, separate the layers and extract with ether, combine the organic phas...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com