Insulin amyloid polypeptide inhibitor, preparation method and application thereof
A technology of amyloid polypeptide and inhibitor, applied in the field of polypeptide compounds for the treatment of diabetes
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0011] Chemical Synthesis of Peptides
[0012] Inhibitor 2 was prepared by Fmoc-protected solid-phase synthesis technique. The synthesis reaction was carried out from the C-terminus to the N-terminus according to the inhibitor 2 sequence, and there were free amino groups on Rink amide MBHA resin and Fmoc-Gly-Wang resin as carriers (available from Advanced Chem Tech). During each ligation step, the amino acid residues are activated, and there are 4 times as many HBTU, HOBt, DIEA and Fmoc-amino acids as there are free amino groups on the carrier in the activation mixture. Use 6-chloro-1-hydroxybenzotriazole and N,N-diisopropylcarbodiimide as condensing agents to carry out connection reaction. After each amino acid connection reaction, use pyridine / acetic acid / N-methyl A mixture of imidazole (4:1:0.5) was used to block the unconnected free amino group, and the blocking reaction was 10 minutes. Before the next amino acid is connected, the Fmoc-group on the carrier must be remove...
Embodiment 2
[0015] In Vitro Apoptosis Assay by Amylin Inhibitor 2
[0016] In this experiment, the effect of insulin amyloid peptide inhibitor 2 on inhibiting the apoptosis of insulinoma cells (INS-1 cells) was studied. After the cells were treated with 10 μmol / L insulin-like amyloid polypeptide, many green bright spots appeared after TUNEL staining compared with the medium control group, indicating that there were apoptotic cells in the system. When 20 μmol / L insulin-amyloid peptide inhibitor 2 was co-incubated with insulin-amyloid peptide, microscopic examination showed fewer green highlights than the control group with insulin-like amyloid peptide alone. After counting and counting the photographs, it was found that the apoptosis rate of cells treated with 10 μmol / L insulin-like amyloid polypeptide was about 65.0%, while the apoptosis rate of cells treated with 20 μmol / L inhibitor 2 and insulin-like amyloid polypeptide was reduced to 30.2% (p<0.05). And there was no significant diffe...
Embodiment 3
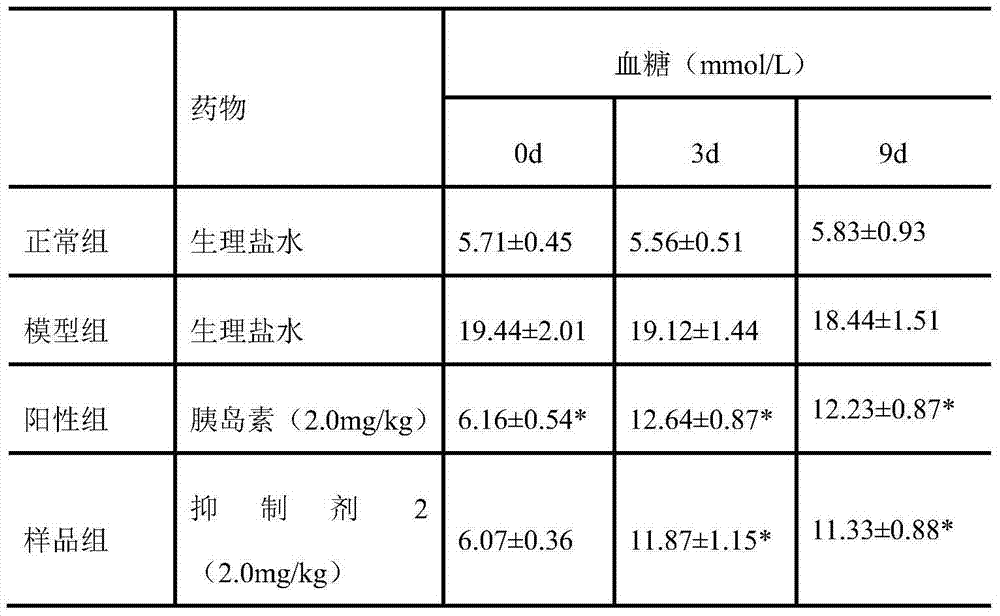
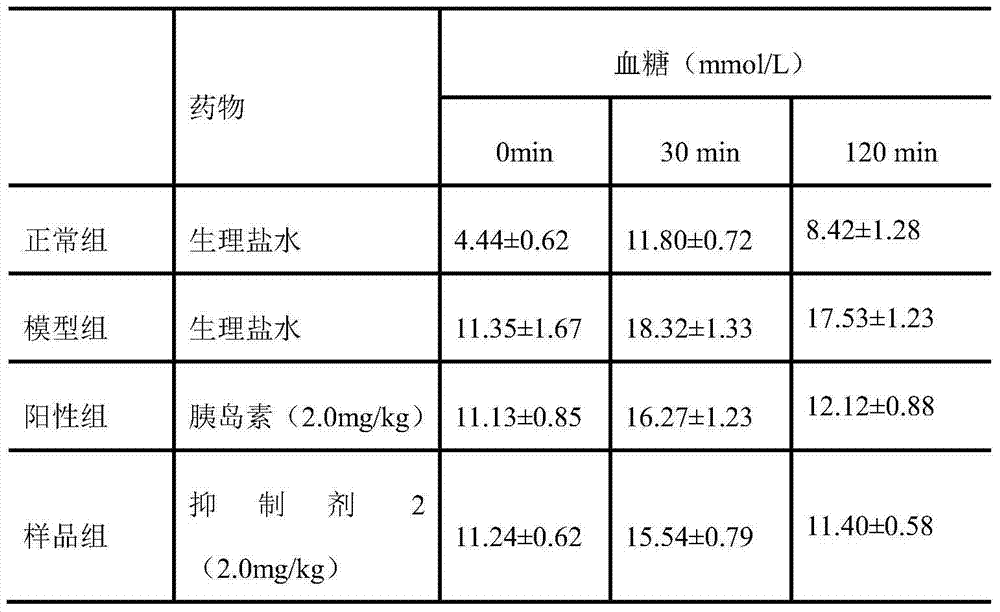
[0018] In vivo hypoglycemic experiment of amylin inhibitor 2
[0019] Kunming mice were fed with high-fat and high-sugar to a body weight of 18-22 g, half male and half female, fasted for 24 hours before the experiment, and intraperitoneally injected with streptozotocin (STZ) 50 mg / kg (1% citrate buffer). liquid solution preparation). One week later, blood glucose (BS) was measured by blood collection from the tail vein of the mice. If the BS was higher than 16mmol / L, the modeling was successful. After modeling, the mice were divided into 3 groups, 10 in each group. Diabetes model group (DM group): subcutaneous injection of the same amount of PBS buffer; control group (insulin): 20 μg / kg subcutaneous injection (dissolved in 0.1mol / L PBS buffer), twice a day for 9 consecutive days; sample group (Amylin inhibitor 2): the same as the control group; another 10 normal mice were taken as the blank group. On the 9th day after administration, blood was taken from the tail vein to me...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com