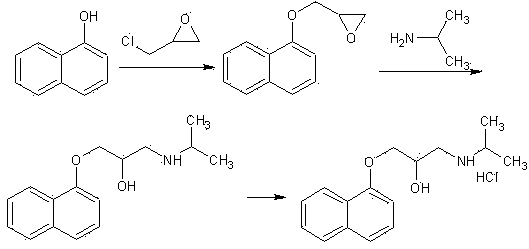
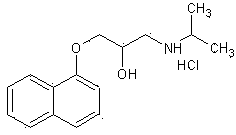
Improved preparation method of propranolol
A naphthyloxy and organic solvent technology, applied in the field of improved preparation of propranolol, can solve the problems of poor recovery effect, low recovery rate, easy loss and the like, and achieve the effects of less pollution, less side reactions, and less three wastes
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] Weigh 200.2g of 3-(1-naphthyloxy)-1,2-propylene oxide, 70.9g of isopropylamine, Hβ molecular sieve (SiO 2 / Al 2 o 3 = 60) 4.0g and 1200ml of dichloromethane in a reaction flask. Stir the reaction at 30-35°C. The reaction was monitored by TLC. After 110 minutes, the reaction was stopped. The catalyst was removed by filtration, the filtrate was evaporated to dryness under reduced pressure, and the residue was recrystallized from n-hexane to obtain 239.4 g of propranolol with a yield of 92.3% and a purity of 99.2% by HPLC.
Embodiment 2
[0029] Weigh 200.2g of 3-(1-naphthyloxy)-1,2-propylene oxide, 76.8g of isopropylamine, Hβ molecular sieve (SiO 2 / Al 2 o 3 = 60) 2.0g and 1400ml of dichloromethane in a reaction flask. Stir the reaction at 25-30°C. The reaction was monitored by TLC. After 120 minutes, the reaction was stopped. The catalyst was removed by filtration, the filtrate was evaporated to dryness under reduced pressure, and the residue was recrystallized from n-hexane to obtain 241.2 g of propranolol with a yield of 93.0% and an HPLC purity of 99.1%.
Embodiment 3
[0031] Weigh 200.2 g of 3-(1-naphthyloxy)-1,2-propylene oxide, 59.1 g of isopropylamine, Hβ molecular sieve (SiO 2 / Al 2 o 3 = 60) 4.0g and 1200ml of dichloromethane in a reaction flask. Stir the reaction at 25-30°C. The reaction was monitored by TLC. After 130 minutes, the reaction was stopped. The catalyst was removed by filtration, the filtrate was evaporated to dryness under reduced pressure, and the residue was recrystallized from n-hexane to obtain 240.4 g of propranolol with a yield of 92.7% and an HPLC purity of 99.2%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com