Preparation method of perampanel intermediate
A technology for intermediate and pyridine is applied in the field of preparation of perampanel intermediate 5--2-pyridone, which can solve the problems of unfavorable industrialized production, few preparation methods, complicated operation and the like, and achieves shortening of production cycle and process route. The effect of novel and simplified process operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
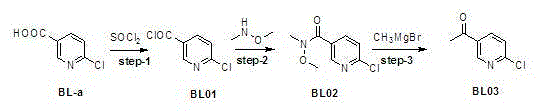
[0028] Example 1: Synthesis of 6-chloronicotinyl chloride (compound BL01)
[0029] Add 1.4kg of 6-chloronicotinic acid into 7L of toluene, stir and disperse. Then add 4.2Kg of thionyl chloride, heat to 80-90°C, and react for 10 hours. After the reaction was complete, it was concentrated to dryness under reduced pressure to obtain compound BL01, which was kept for future use.
Embodiment 2
[0030] Example 2: Synthesis of N-methoxy-N-methyl-6-chloronicotinamide (compound BL02)
[0031] 1.5 kg of dimethylhydroxylamine hydrochloride was dispersed in 10 L of dichloromethane, and the 6-nicotinoyl chloride obtained in Example 1 was slowly added dropwise into the reaction system, and reacted for 1 hour. After the reaction was completed, it was washed with water and saturated sodium chloride successively. It was dried over anhydrous sodium sulfate and concentrated under reduced pressure to obtain 1.65kg of compound BL02.
[0032] 1 H NMR (CDCl 3 , δ ppm): 3.39 (s, 3H), 3.73 (s, 3H), 7.32-7.37 (m, 1H), 7.73-7.80 (m, 1H), 8.59-8.61 (m, 1H); MS (ES+) m / z 210.6.
Embodiment 3
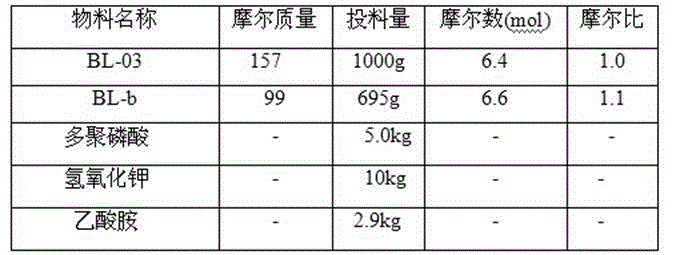
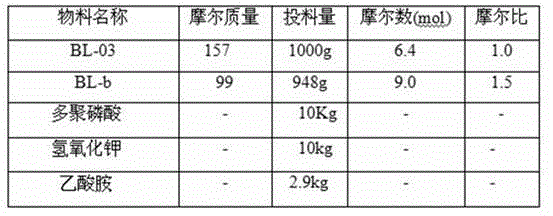
[0033] The synthesis of embodiment 3,2-chloro-5-acetylpyridine (compound BL03)
[0034] Disperse 1.6kg of compound (BL02) in 8L of tertiary methyl ether, and add 3L of methylmagnesium chloride solution dropwise. React for 1 hour. Stop the reaction, pour the reaction solution into 10L of water, and extract with dichloromethane. The organic phase was washed successively with water and saturated sodium chloride, dried over anhydrous sodium sulfate, and concentrated under reduced pressure to obtain 1.2kg of compound BL03.
[0035] 1 H NMR (CDCl 3 , δ ppm): 3.98 (s, 3H), 7.34-7.39 (m, 1H), 7.65-7.76 (m, 1H), 8.59-8.61 (m, 1H); MS (ES+) m / z 157.1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com