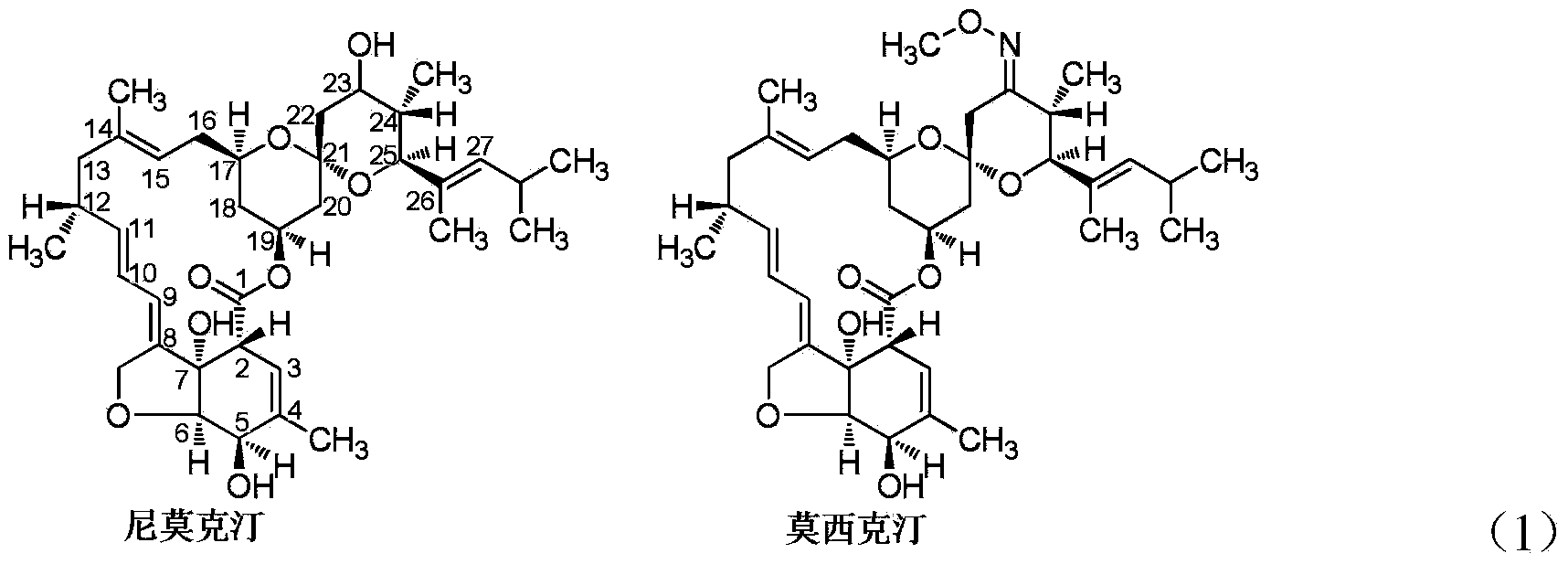
Method for separating and preparing high-purity Moxidectin membrane
A moxictine and high-purity technology, applied in the field of membrane separation and preparation of high-purity moxictine, can solve the problems of high energy consumption, high investment cost and production cost, complicated process, etc., and achieves low energy consumption and reduced Production cost and investment cost, the effect of improving product purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] The steps of the method for preparing high-purity moxictine in this embodiment are as follows:
[0029] (1) The fermented liquid produced by Streptomyces fermentation undergoes solid-liquid separation, and the pre-purified liquid of nemoctine obtained after leaching has a purity of 15.6%, which contains 80 g of solids with a concentration of 1.5%, and is pumped into polyethersulfone hollow fiber microfiltration Membrane filtration, control pump pressure 0.1-0.7MP. The membrane filtrate was received to obtain a filtrate with a purity of 20%.
[0030] The above-mentioned filtrate is pumped into a roll-type ultrafiltration membrane with a molecular weight cut-off of 3000 for filtration, and the pump pressure is controlled at 0.5-0.9MP to receive the filtrate to obtain a filtrate with a purity of 52.5%.
[0031] (2) The nimoctine solution after ultrafiltration in step (1) is pumped into a roll-type nanofiltration membrane with a molecular weight cut off of 200 for concentr...
Embodiment 2
[0038] The steps of the method for preparing high-purity moxictine in this embodiment are as follows:
[0039] (1) The fermented liquid produced by Streptomyces fermentation is subjected to solid-liquid separation, and the pre-purified liquid of nemoctine obtained after leaching has a purity of 18.2%, which contains 90 g of solids with a concentration of 1.8%, and is pumped into polyethersulfone hollow fiber microfiltration Membrane filtration, control pump pressure 0.1-0.7MP. The membrane filtrate was received to obtain a filtrate with a purity of 20%.
[0040] The above-mentioned filtrate is pumped into a roll-type ultrafiltration membrane with a molecular weight cut-off of 2000 for filtration, and the pump pressure is controlled at 0.7-1.2MP to receive the filtrate to obtain a filtrate with a purity of 52%.
[0041] (2) The nimoctine solution after ultrafiltration in step (1) is pumped into a roll-type nanofiltration membrane with a molecular weight cut off of 200 for conc...
Embodiment 3
[0048] The steps of the method for preparing high-purity moxictine in this embodiment are as follows:
[0049] (1) The fermented liquid produced by Streptomyces fermentation is subjected to solid-liquid separation, and the pre-purified liquid of nimoctine obtained after leaching has a purity of 13.2%, which contains 100 g of solids with a concentration of 1.4.8%, and is pumped into the polyethersulfone central control Fiber microfiltration membrane filtration, control pump pressure 0.1-0.7MP. The membrane filtrate was received to obtain a filtrate with a purity of 18.6%.
[0050] The above-mentioned filtrate is pumped into a roll-type ultrafiltration membrane with a molecular weight cut-off of 1500 for filtration, and the pump pressure is controlled at 0.7-1.2MP to receive the filtrate to obtain a filtrate with a purity of 51.4%.
[0051] (2) The nimoctine solution after ultrafiltration in step (1) is pumped into a roll-type nanofiltration membrane with a molecular weight cut...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com