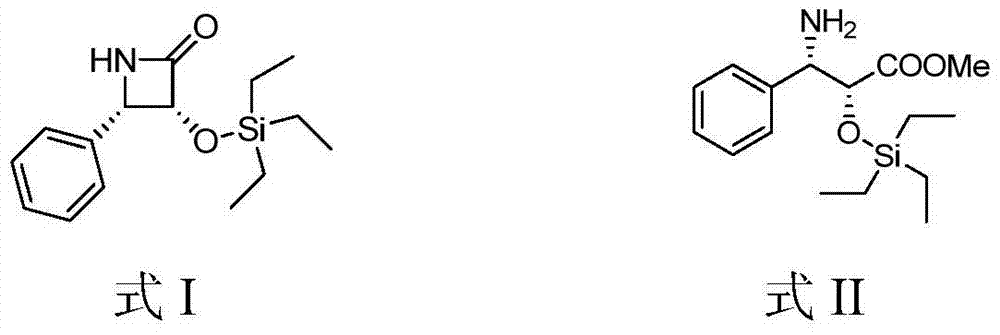A kind of preparation method of four-membered ring taxane side chain compound
A compound and taxane technology, applied in the field of preparation of four-membered ring taxane side chain compounds, can solve the problems of unsuitability for industrial production, low reaction yield, cumbersome post-treatment, etc., and achieve easy industrial production and harvest The effect of high efficiency and simple equipment
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
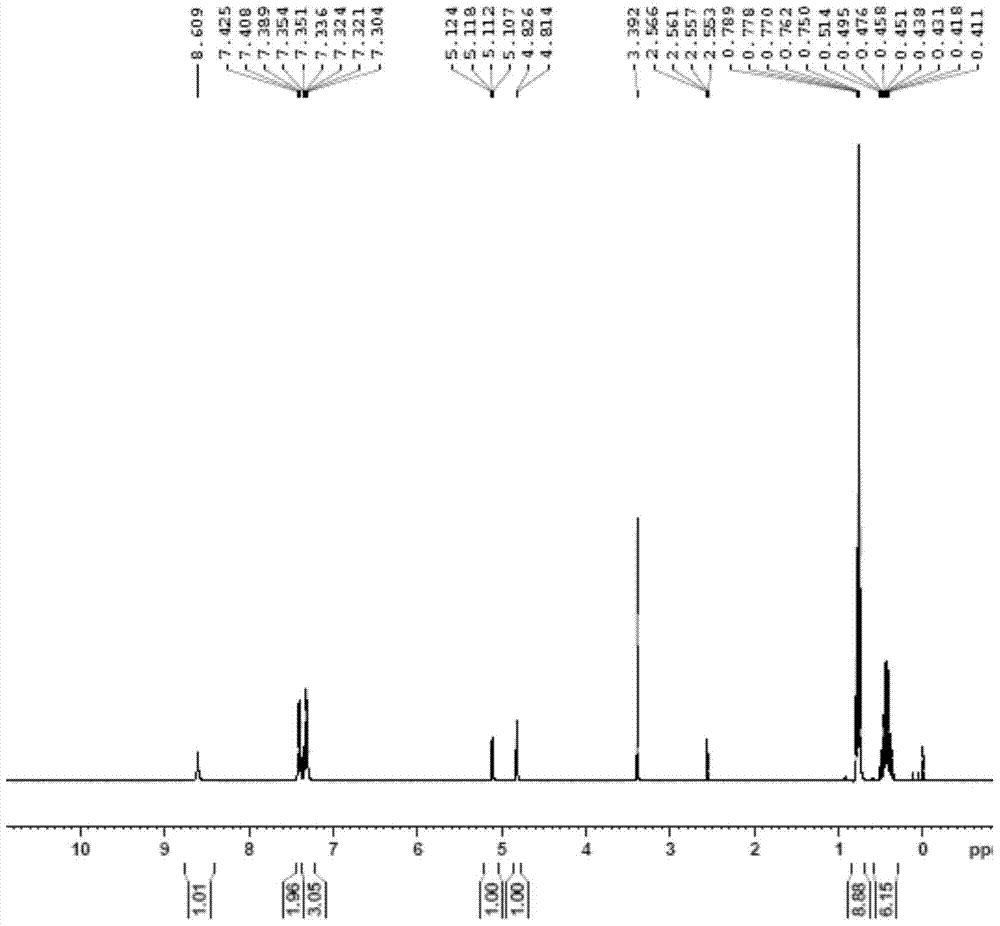
[0023] The compound shown by formula II (2mmol) and lithium chloride (0.2mmol) were dissolved in tetrahydrofuran (20mL) respectively, put into an ice-water bath, and after cooling to -30°C, lithium hexamethyldiaminesilyl ( 2mmol), after reacting for 1 hour, TLC showed that the raw materials were completely reacted, the reaction solution was poured into a saturated ammonium chloride aqueous solution, extracted with ethyl acetate, dried over anhydrous sodium sulfate, suction filtered and concentrated to obtain a crude product. The crude product is crystallized with sherwood oil / ethyl acetate to obtain the refined product, and the yield is 34%. figure 1 shown.
[0024] The control of the reaction temperature in this embodiment is completed under the protection of nitrogen; in addition, the catalyst—lithium chloride can also be added simultaneously with the base—hexamethyldiaminosilyllithium.
[0025] 1H-NMR (400MHz, d6-DMSO): 0.44 (m, 6H); 0.76 (t, J = 7.9, 9H); 4.79 (d, J = 4.7...
Embodiment 2
[0028] The compound shown by formula II (2mmol) and lithium chloride (1.8mmol) were added to tetrahydrofuran (20mL) to dissolve respectively. At 25°C, lithium hexamethyldiamide silicon base (20mmol) was added, and after 8 hours of reaction, TLC It showed that the reaction of raw materials was complete, the reaction solution was poured into saturated ammonium chloride aqueous solution, extracted with ethyl acetate, dried over anhydrous sodium sulfate, filtered with suction and concentrated to obtain a crude product. The crude product is crystallized with sherwood oil / ethyl acetate to obtain the refined product, and the yield is 45%. figure 1 shown.
[0029] The control of the reaction temperature in this embodiment is completed under the protection of nitrogen; in addition, the catalyst—lithium chloride can also be added simultaneously with the base—hexamethyldiaminosilyllithium.
[0030]
Embodiment 3
[0032] The compound shown by formula II (2mmol) and lithium chloride (0.4mmol) were added to tetrahydrofuran (20mL) to dissolve respectively, put into an ice-water bath, and after cooling to 0°C, hexamethyldiaminosilyllithium (8mmol ), after reacting for 4 hours, TLC showed that the raw material had reacted completely, and the reaction solution was poured into a saturated ammonium chloride aqueous solution, extracted with ethyl acetate, dried over anhydrous sodium sulfate, suction filtered and concentrated to obtain a crude product. The crude product is crystallized with petroleum ether / ethyl acetate to obtain a refined product, and the yield is 91%. figure 1 shown.
[0033] The control of the reaction temperature in this embodiment is completed under the protection of nitrogen; in addition, the catalyst—lithium chloride can also be added simultaneously with the base—hexamethyldiaminosilyllithium.
[0034]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com