High-color-purity red luminescent material for blue light excitation and preparation method of red luminescent material
A technology of red light emission and blue light excitation, applied in the direction of light-emitting materials, chemical instruments and methods, etc., can solve the problems of low luminous efficiency, low absorption efficiency, low luminous efficiency of red phosphor powder, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0014] Weigh barium carbonate (BaCO 3 ): 0.996 g, germanium oxide (GeO2): 0.523 g, potassium permanganate (KMnO 4 ): 0.063g, then add 5 mL (40 %) hydrofluoric acid (HF) to the above solid mixture and stir to dissolve, then add 45 mL of distilled water. Then the resulting solution was reacted in an autoclave at 180°C for 12 hours, cooled to room temperature, washed with distilled water, and then dried in a vacuum oven for 24 hours. The final pink powder obtained was the final BaGeF 6 :Mn 4+ Phosphor.
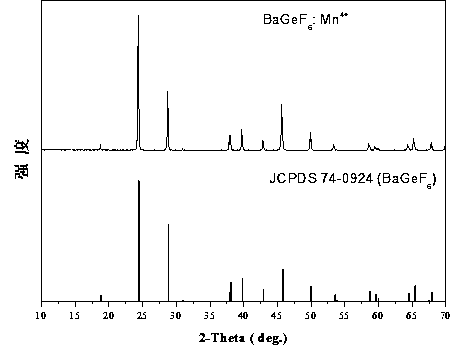
[0015] The XRD diffraction pattern of this fluorescent powder is attached figure 1 shown, with the standard card JCPDS 74-0924 (BaGeF 6 ) in contrast, the two are completely consistent, and no diffraction peaks of any heterogeneous phases are observed, which indicates that the samples we synthesized have a single crystal phase.
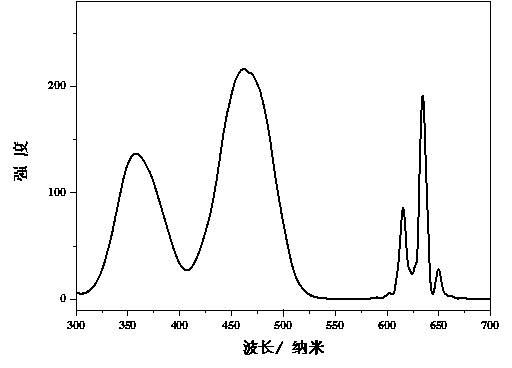
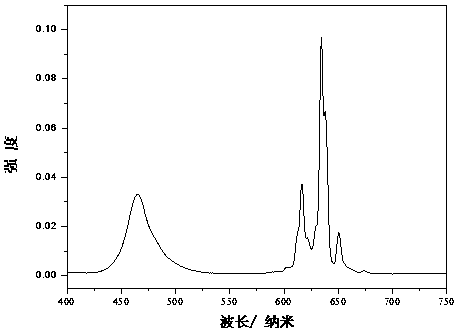
[0016] attached figure 2 Shown are the room temperature excitation spectrum (monitored at 634 nm) and emission spectrum (excited at 460 nm) of th...
Embodiment 2
[0019] Weigh barium carbonate (BaCO 3 ): 0.996 g, germanium oxide (GeO 2 ): 0.523 g, potassium permanganate (KMnO 4 ): 0.063g, then add 10 mL (40 %) hydrofluoric acid (HF) to the above solid mixture and stir to dissolve, then add 40 mL of distilled water. Subsequently, the resulting solution was reacted in an autoclave at 180°C for 8 hours, cooled to room temperature, washed with distilled water, and then dried in a vacuum oven for 24 hours. Finally, the pink powder obtained was the final BaGeF 6 :Mn 4+Phosphor.
Embodiment 3
[0021] Weigh barium carbonate (BaCO 3 ): 0.996 g, germanium oxide (GeO 2 ): 0.523 g, potassium permanganate (KMnO 4 ): 0.033 g, then add 10 mL (40%) hydrofluoric acid (HF) to the above solid mixture and stir to dissolve, then add 40 mL of distilled water. Subsequently, the resulting solution was reacted in an autoclave at 180°C for 8 hours, cooled to room temperature, washed with distilled water, and then dried in a vacuum oven for 24 hours. Finally, the pink powder obtained was the final BaGeF 6 :Mn 4+ Phosphor.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com