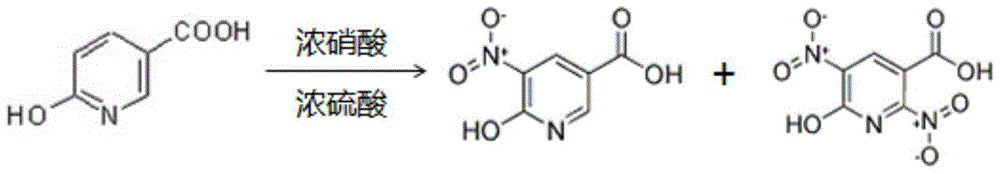
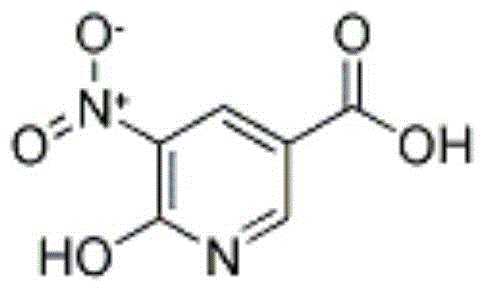
A kind of synthetic method of 6-hydroxy-5-nitronicotinic acid and its separation and purification method
A technology for the synthesis of nitronicotinic acid, which is applied in the direction of organic chemistry, can solve the problems of difficult separation and purification, low yield, and many by-products in the reaction of picolinic acid compounds, and achieve the effect of increasing conversion rate and promoting conversion
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] A method for synthesizing 6-hydroxy-5-nitronicotinic acid, the steps are:
[0035] (a) Add concentrated sulfuric acid and concentrated nitric acid to the reaction flask in sequence and stir evenly to obtain reaction solution A. The molar ratio of concentrated sulfuric acid to concentrated nitric acid is 1:6, and the mass fraction of concentrated sulfuric acid used is 68%. The mass score is 86%;
[0036] (b) Mixing the raw material 6-hydroxynicotinic acid and the catalyst ammonium bisulfate according to a molar ratio of 50:1 to obtain reaction raw material B;
[0037] (c) Under stirring conditions, add the reaction material B obtained in step (b) to the reaction solution A obtained in step (a), and then heat to 60°C for a pre-nitrification reaction for 4 hours, wherein the HNO in the reaction solution A 3 The molar ratio with 6-hydroxynicotinic acid in the reaction raw material B is 1.5:1;
[0038] (d) Mix concentrated sulfuric acid and concentrated nitric acid (the mass fractio...
Embodiment 2
[0046] A method for synthesizing 6-hydroxy-5-nitronicotinic acid, the steps are:
[0047] (a) Add concentrated sulfuric acid and concentrated nitric acid to the reaction flask in sequence and stir evenly to obtain reaction solution A. The molar ratio of concentrated sulfuric acid to concentrated nitric acid is 1:5, and the mass fraction of concentrated sulfuric acid used is 68%. The mass score is 86%;
[0048] (b) Mixing the raw material 6-hydroxynicotinic acid and the catalyst ammonium bisulfate at a molar ratio of 60:1 to obtain reaction raw material B;
[0049] (c) Under stirring conditions, add the reaction material B obtained in step (b) to the reaction solution A obtained in step (a), and then heat to 50°C for a pre-nitrification reaction for 5 hours, wherein the HNO in the reaction solution A 3 The molar ratio with 6-hydroxynicotinic acid in the reaction raw material B is 1.0:1;
[0050] (d) Mix the concentrated sulfuric acid and concentrated nitric acid (the mass fraction of c...
Embodiment 3
[0058] A method for synthesizing 6-hydroxy-5-nitronicotinic acid, the steps are:
[0059] (a) Add concentrated sulfuric acid and concentrated nitric acid to the reaction flask in sequence and stir evenly to obtain reaction solution A. The molar ratio of concentrated sulfuric acid to concentrated nitric acid is 1:7, and the mass fraction of concentrated sulfuric acid used is 68%. The mass score is 86%;
[0060] (b) Mixing the raw material 6-hydroxynicotinic acid and the catalyst ammonium bisulfate at a molar ratio of 70:1 to obtain reaction raw material B;
[0061] (c) Under stirring conditions, add the reaction material B obtained in step (b) to the reaction solution A obtained in step (a), and then heat to 65° C. for pre-nitration reaction for 4.5 hours, wherein the reaction solution A in HNO 3 The molar ratio with 6-hydroxynicotinic acid in the reaction raw material B is 1.3:1;
[0062] (d) Mix concentrated sulfuric acid and concentrated nitric acid (the mass fraction of concentrat...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com