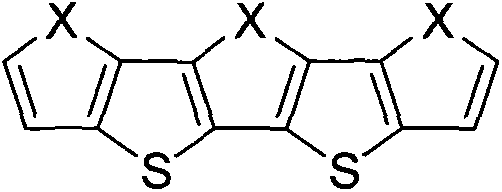
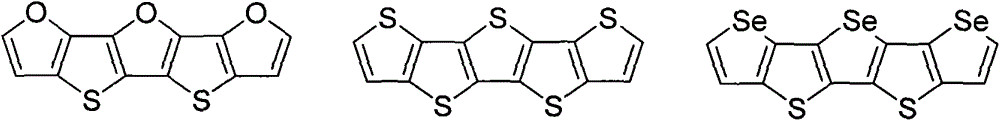
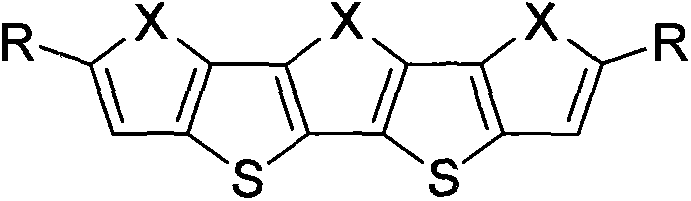
Oxygen family element-containing penta-condensed ring conjugated molecule and its derivative synthetic method and purpose thereof
A technology of conjugated molecules and oxygen group elements, applied in electrical components, photovoltaic power generation, circuits, etc., can solve problems such as poor stability, strong absorption in the visible light region, and restrictions on the development of displays, and achieve good solubility and high carrier migration rate effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0043] Preparation of:
[0044] At -78°C, add 1 part of 3-bromofuran into anhydrous THF, add 1 part of BuLi dropwise, stir for 0.5-3 hours, and then move to room temperature. Add 1 to 2.0 parts of dimethyl disulfide to the above mixture, stir for 10 to 24 hours, quench with saturated ammonium chloride, extract with dichloromethane, dry and spin dry, and pass through a column to obtain 3-thiomethylfuran.
[0045] At 0°C, add 1 part of 3-thiomethylfuran into dichloromethane, add 1 part of bromine, stir for 2.5-5.0 hours, add saturated NaHSO 3 Or sodium thiosulfate solution, extract the organic phase and dry it, and pass through the column to obtain 2-bromo-3-thiomethylfuran
[0046] Take 2.2-3.0 parts of 2-bromo-3-thiomethylfuran and add it to THF, 2 Or add 2 to 10% Pd(PPh under the protection of Ar 3 ) 4 , Heated to reflux for 24 ~ 48h. The THF was spun out, the organic phase was extracted and dried, and passed through the column to obtain 2,5-(2-(3-thiomethyl)furan)fura...
example 2
[0049] Preparation of:
[0050] At -78°C, add 1 part of 3-bromothiophene to anhydrous THF, add 1 part of BuLi dropwise, stir for 0.5-3 hours, and then move to room temperature. Add 1 to 2.0 parts of dimethyl disulfide to the above mixture, stir for 10 to 24 hours, quench with saturated ammonium chloride, extract with dichloromethane, dry and spin dry, and pass through a column to obtain 3-thiomethylthiophene.
[0051] At 0°C, add 1 part of 3-thiomethylthiophene to dichloromethane, add 1 part of bromine, stir for 2.5-5.0 hours, add saturated NaHSO 3 Or sodium thiosulfate solution, extract the organic phase, dry and spin dry, and pass through the column to obtain 2-bromo-3-thiomethylthiophene.
[0052] Take 2.2-3.0 parts of 2-bromo-3-thiomethylthiophene and add it to THF, 2 Or add 2 to 10% Pd(PPh under the protection of Ar 3 ) 4 , Heated to reflux for 24 ~ 48h. The THF was spun out, and the organic phase was obtained by extraction, which was dried and spin-dried, and pass...
example 3
[0055] Preparation of:
[0056] At -78°C, add 1 part of 3-bromoselenophene to anhydrous THF, add 1 part of BuLi dropwise, stir for 0.5-3 hours and then move to room temperature. Add 1 to 2.0 parts of dimethyl disulfide to the above mixture, stir for 10 to 24 hours, quench with saturated ammonium chloride, extract with dichloromethane, dry and spin dry, and pass through a column to obtain 3-thiomethylthiophene.
[0057] At 0°C, add 1 part of 3-thiomethylselenophene to dichloromethane, add 1 part of bromine, stir for 2.5-5.0 hours, add saturated NaHSO 3 Or sodium thiosulfate solution, the organic phase was extracted and dried, and passed through the column to obtain 2-bromo-3-thiomethylselenophene.
[0058] Take 2.2-3.0 parts of 2-bromo-3-thiomethylselenophene and add it to THF, 2 Or add 2-10% Pd(PPh under the protection of Ar 3 ) 4, heating to reflux for 24 to 48 hours. The THF was spun out, the organic phase was extracted and dried, and passed through the column to obtai...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com