Triazole Cd(II) complex with two-dimensional planar porous framework structure and synthesis method and application thereof
A two-dimensional planar, porous framework technology, applied in the direction of 2/12 organic compounds without C-metal bonds, organic chemical methods, cadmium organic compounds, etc., can solve the adsorption performance of various polycyclic aromatic hydrocarbons, which has not been reported And other issues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] Synthesis of Complex (1)
[0027] Weigh 0.2097g (0.5 mmol) Cd(ClO 4 ) 2 ·6H 2 Dissolve O in 5 mL of water, weigh 0.089 g (0.5 mmol) of ligand L (L can be purchased through Alfa reagents) and dissolve it in 10 mL of ethanol, and add 0.005 g of ascorbic acid at the same time; mix the above two solutions, and then add 0.076 g (1 mmol) NH 4 After adding NCS, stir at room temperature for about two hours; filter the solution and let it stand, two months later grow colorless transparent crystals.
[0028] Elemental analysis results, experimental value (%): C, 30.52 %; H, 3.81 %; N, 15.83 %;
[0029] According to C 18 h 26 CdCl 2 N 8 o 11 Calculated theoretical values (%): C, 30.29 %; H, 3.67 %; N, 15.70 %.
[0030] FT-IR (KBr, cm -1 ): 3460 (br), 3258 (m), 3135 (w), 1613 (m), 1519 (m), 1432 (m), 1375 (m), 1276 (m), 1100 (bs), 1017 (m ), 975 (m), 930 (m), 819 (m), 766 (m), 708 (m), 634 (s), 543 (m).
Embodiment 2
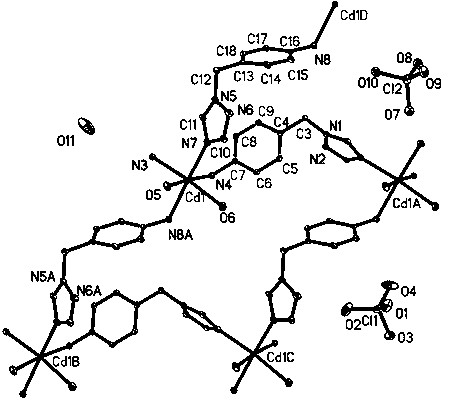
[0032] (1) Structure determination of complexes
[0033] A single crystal with a size of 0.18 mm × 0.17 mm × 0.16 mm was selected using a BRUKER SMART 1000 X-ray single crystal diffractometer, and Mo K alpha radiation ( lambda = 0.071 073 nm) as a diffraction light source, at 293 (2) K temperature, with φ - ω scan mode, in 1.47 θ ≤ θ ≤ 25.01 θ (-11≤ h ≤11, -12≤ k ≤ 12, -17 ≤ l ≤ 11), a total of 7859 diffraction points were collected, of which 4745 independent diffraction points [I > 2 α(I)](R int = 0.0177). The crystal structure was solved by the direct method, and the non-hydrogen atoms were obtained by the difference Fourier synthesis method. The method of determining and correcting the hydrogen atoms was theoretical hydrogenation, and the isotropic and anisotropic thermal parameters were used for the hydrogen atoms and non-hydrogen atoms. The structure is corrected by the full matrix least squares method, and all calculations are completed with SHELXS-9...
Embodiment 3
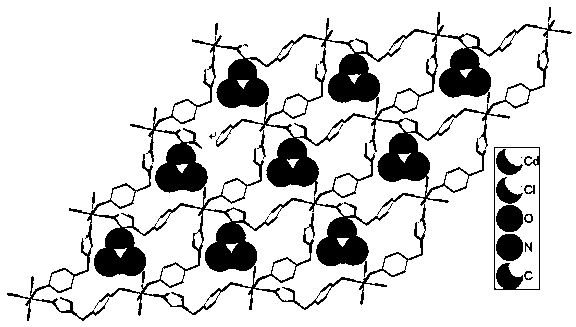
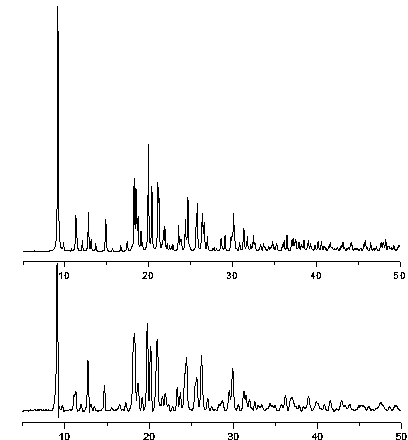
[0049] {[Cd(μ 2 -L) 2 (H 2 O) 2 ](ClO 4 ) 2 ·H 2 O} (1) compounds are used as pre-concentration materials, and are applied in the detection and analysis of environmental organic pollutants
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com