Method for detecting content of water-soluble ingredients in salvia miltiorrhiza medicinal material and application of method
A determination method, water-soluble technology, applied in the field of traditional Chinese medicine detection
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
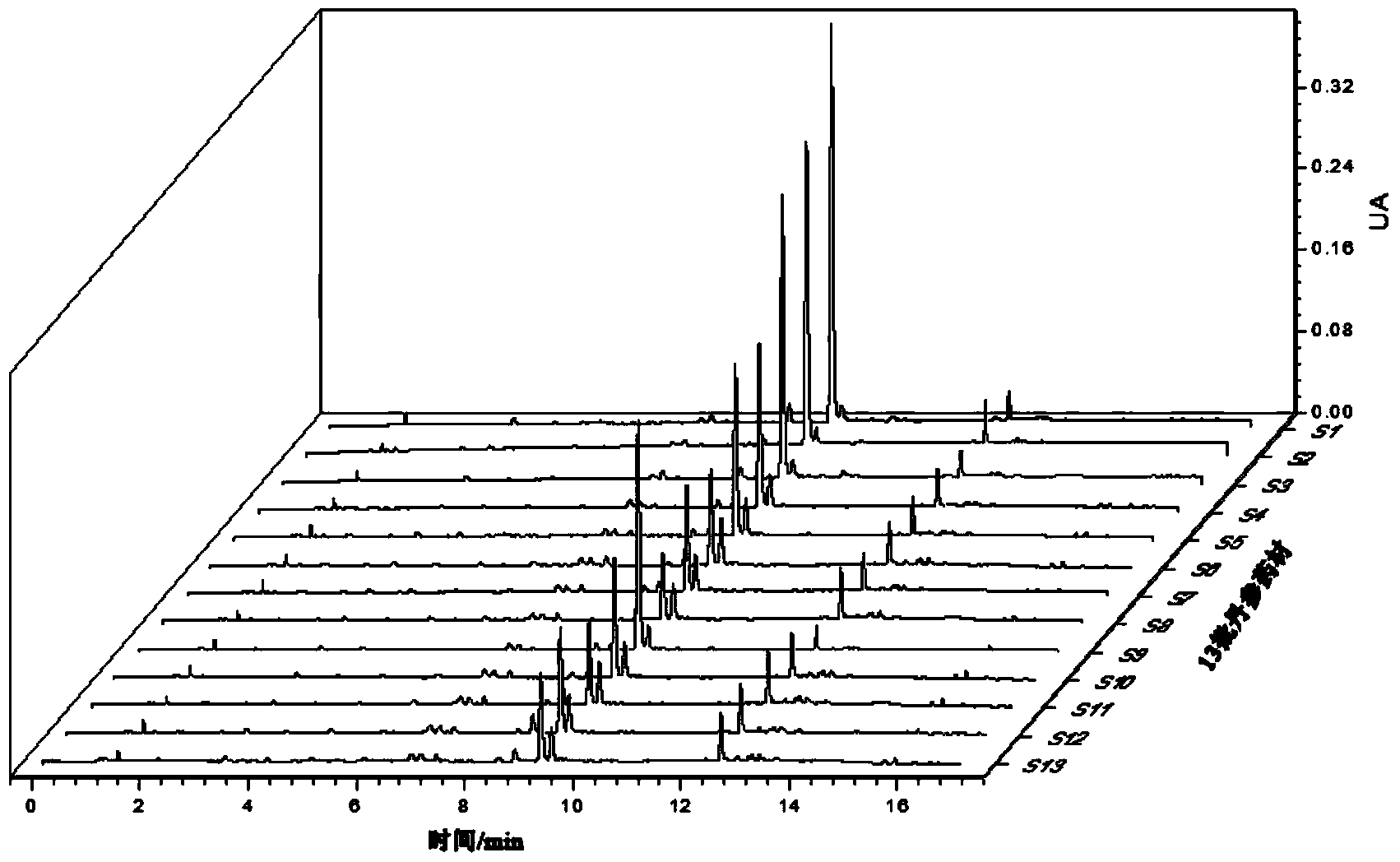
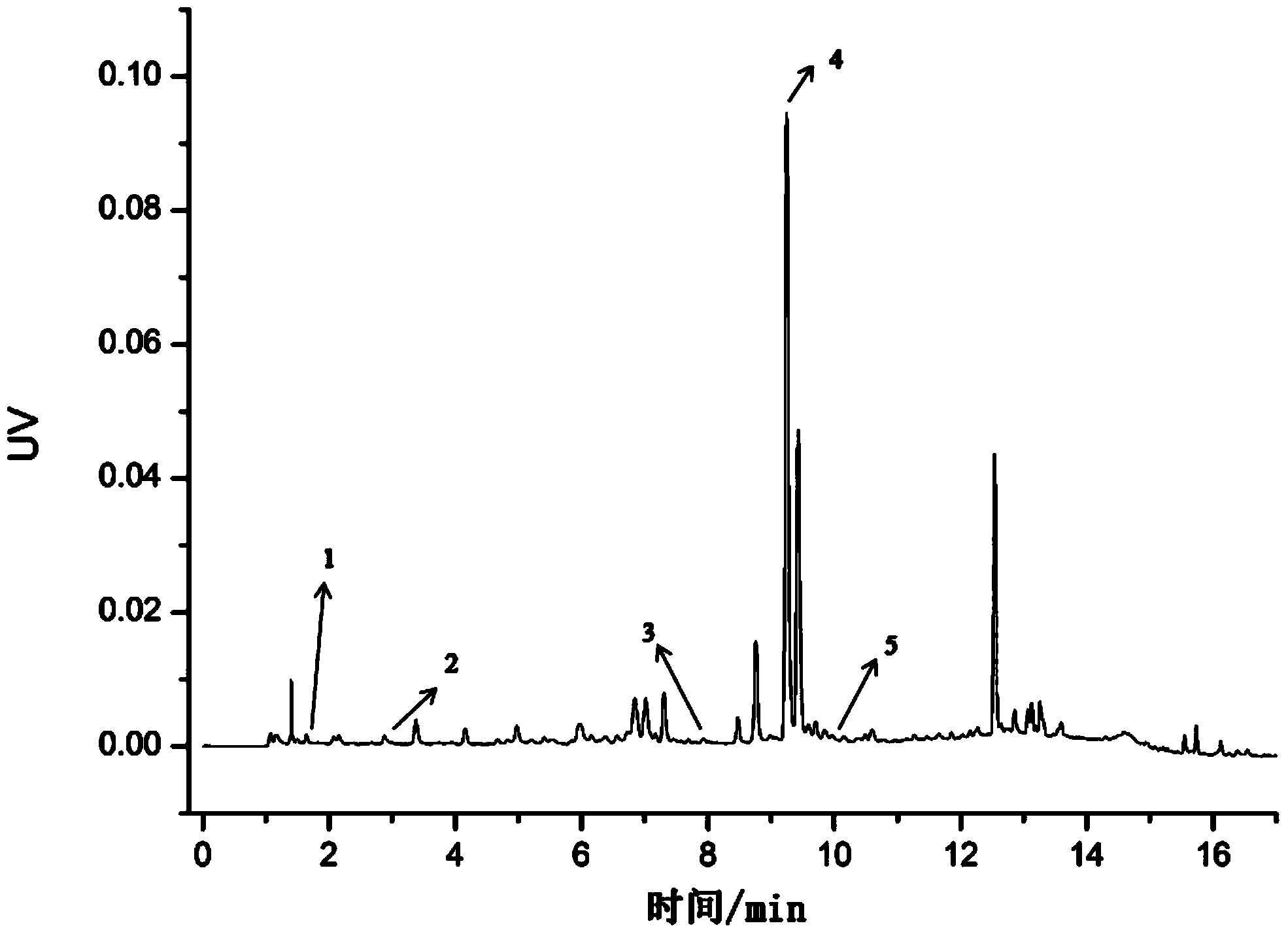
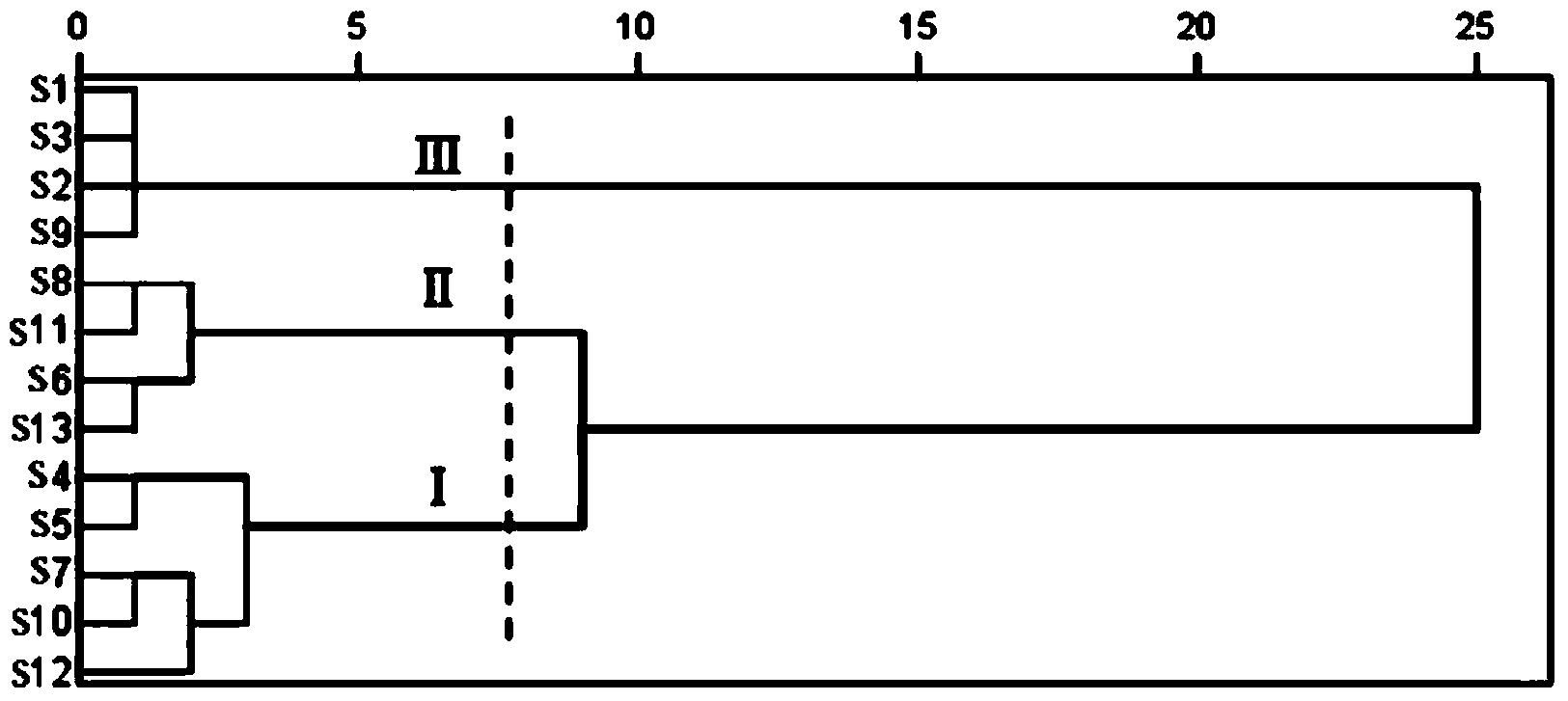
[0098] Embodiment 1: The method for determining the content of water-soluble components in Salvia miltiorrhiza medicinal materials is: according to ultra-high performance liquid chromatography, measure according to the following steps:
[0099] (1) Chromatographic conditions and system suitability test: use octadecylsilane bonded silica gel as filler; use 0.026% phosphoric acid aqueous solution as mobile phase A, acetonitrile as mobile phase B, A: B=85~10:15~ 90, Gradient elution: 0~5min: A: B=85~75:15~25, 5~8min: A:B=75~70:25~30, 8~12min: A:B=70~50: 30~50, 12~12.01min: A:B=50~10:50~90, 12.01~17min: A:B=10:90; detection wavelength is 276nm; flow rate is 0.1mL·min-1; column temperature is 25°C; the number of theoretical plates calculated based on the peak of salvianolic acid B should not be less than 2000;
[0100] (2) Preparation of reference substance solution: take appropriate amount of reference substances of Danshensu, protocatechualdehyde, rosmarinic acid, salvianolic ac...
Embodiment 2
[0103] Embodiment 2: The method for determining the content of water-soluble components in Danshen medicinal materials is: according to ultra-high performance liquid chromatography, measure according to the following steps:
[0104](1) Chromatographic conditions and system suitability test: use octadecylsilane bonded silica gel as filler; use 0.026% phosphoric acid aqueous solution as mobile phase A, acetonitrile as mobile phase B, A: B=85~10:15~ 90, Gradient elution: 0~5min: A: B=85~75:15~25, 5~8min: A:B=75~70:25~30, 8~12min: A:B=70~50: 30~50, 12~12.01min: A:B=50~10:50~90, 12.01~17min: A:B=10:90; detection wavelength is 306nm; flow rate is 0.5mL·min-1; column temperature is 35°C; the number of theoretical plates calculated based on the peak of salvianolic acid B should not be less than 2000;
[0105] (2) Preparation of reference substance solution: take appropriate amount of reference substances of Danshensu, protocatechualdehyde, rosmarinic acid, salvianolic acid B and salv...
Embodiment 3
[0108] Embodiment 3: The determination method of water-soluble component content in Danshen medical material is: according to ultra-high performance liquid chromatography, measure according to the following steps:
[0109] (1) Chromatographic conditions and system suitability test: use octadecylsilane bonded silica gel as filler; use 0.026% phosphoric acid aqueous solution as mobile phase A, acetonitrile as mobile phase B, A: B=85~10:15~ 90, Gradient elution: 0~5min: A: B=85~75:15~25, 5~8min: A:B=75~70:25~30, 8~12min: A:B=70~50: 30~50, 12~12.01min: A:B=50~10:50~90, 12.01~17min: A:B=10:90; detection wavelength is 286nm; flow rate is 0.2mL·min-1; column temperature is 30°C; the number of theoretical plates calculated based on the peak of salvianolic acid B should not be less than 2000;
[0110] (2) Preparation of reference substance solution: take appropriate amount of reference substances of Danshensu, protocatechualdehyde, rosmarinic acid, salvianolic acid B and salvianolic a...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com