1,2,3-Triazole-flavonoid compound-sophocarpidine ternary conjugate and use
A technology of flavonoids and matrine, applied in organic chemistry, drug combination, antineoplastic drugs, etc., can solve the problems of large side effects, large individual differences, and easy metastasis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
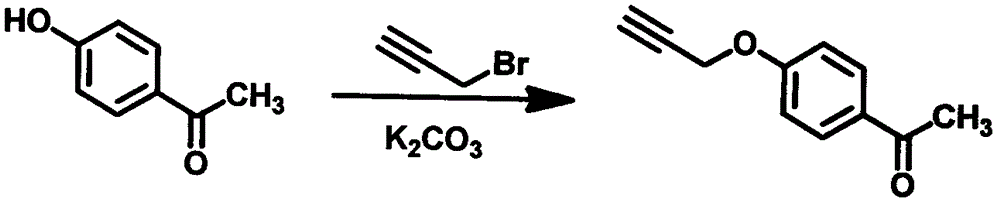
[0053] Example 1 4-propargyloxyacetophenone
[0054] Dissolve 5.0g of 4-hydroxyacetophenone in 50mL of acetone, add 10.2g of anhydrous potassium carbonate, stir at room temperature for 10min, add 6.6g of 80% 3-bromopropynyl toluene solution dropwise under nitrogen protection, stir at 50°C for 2h, filter and take The filtrate was concentrated to obtain a light yellow solid, which was recrystallized from ethanol-petroleum ether to obtain 6.1 g of 4-propargyloxyacetophenone, with a yield of 96% (see figure 1 ).
Embodiment 2
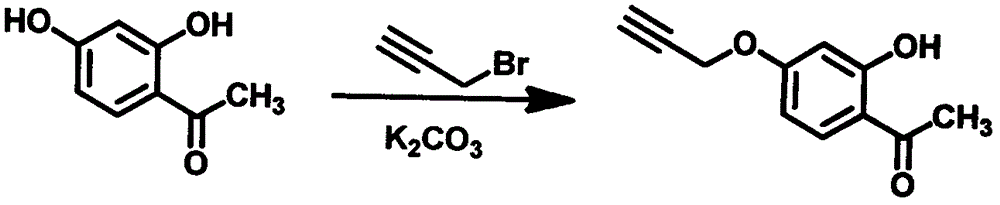
[0055] Example 2 4-propargyloxy-2-hydroxyacetophenone
[0056] Dissolve 25.0 g of 2,4-dihydroxyacetophenone in 350 mL of acetone, add 27.3 g of anhydrous potassium carbonate, stir at room temperature for 10 min, slowly add 23.2 g of 80% 3-bromopropynyl toluene solution dropwise under nitrogen protection, and stir at room temperature for 24 h , filtered to get the filtrate, concentrated to obtain a tan solid, and recrystallized from ethanol-petroleum ether to obtain 24.4g of 4-propargyloxy-2-hydroxyacetophenone, with a yield of 78% (see figure 2 ).
Embodiment 3
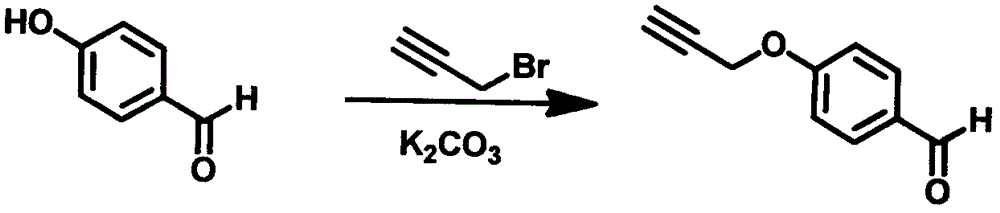
[0057] Example 3 4-propargyloxybenzaldehyde
[0058] Dissolve 5.0g of 4-hydroxybenzaldehyde in 50mL of acetone, add 5.8g of anhydrous potassium carbonate, stir at room temperature for 10min, add dropwise 7.3g of 80% 3-bromopropynyl toluene solution under nitrogen protection, stir at 50°C for 1h, and filter to obtain the filtrate , concentrated to obtain light yellow solid, ethanol-petroleum ether recrystallization, obtain 4-propargyloxybenzaldehyde 5.9g, yield 90% (synthetic route diagram sees image 3 ).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com