Preparation method of weedicide hexazinone
A technology of hexazinone and herbicide, which is applied in the field of preparation of the herbicide hexazinone, can solve the problems of narrow operating flexibility range, potential safety hazards in the use of dimethylamine, strict equipment requirements, etc., and achieves simple synthetic route method and high reaction rate. Effects with low equipment requirements
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
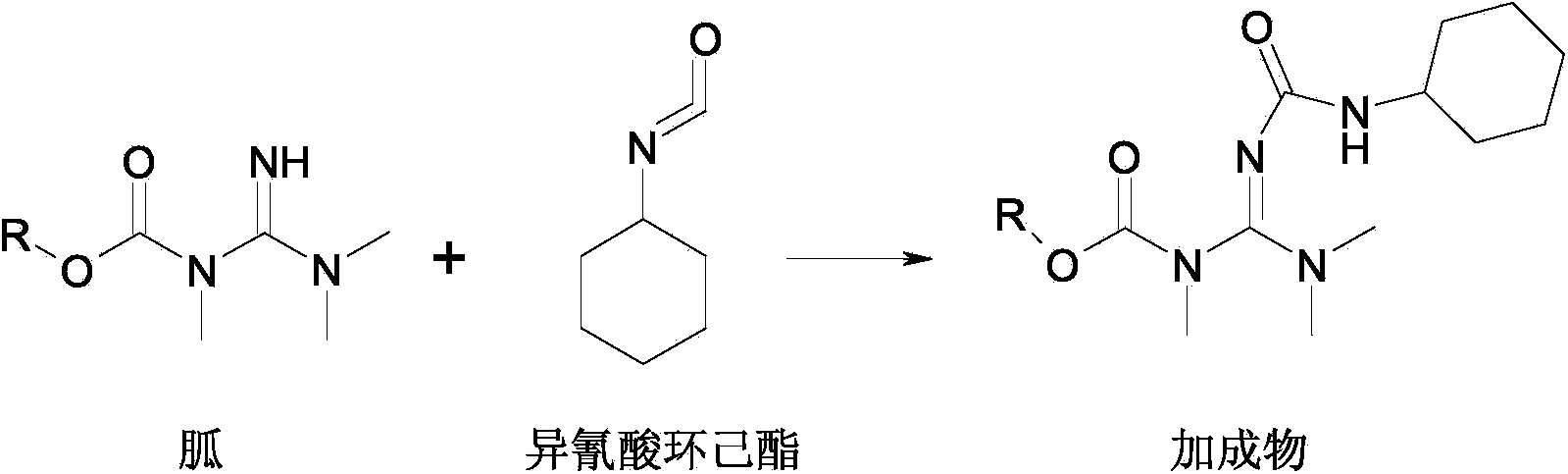
[0028] In the reactant guanidine used in this embodiment, R=ethyl, and the molecular weight is 173.
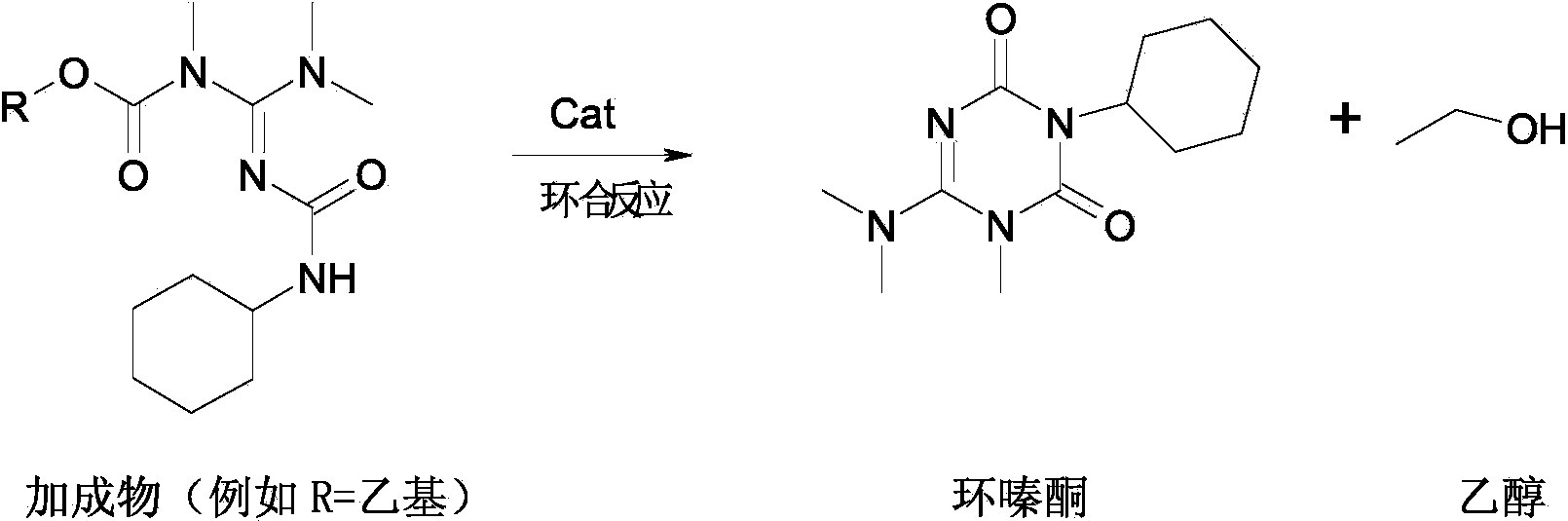
[0029] Mechanical stirring, thermometer and inner diameter 15mm are housed, add the toluene solution 346g of 800g toluene, guanidine in the 2000mL reaction bottle of length 300mm built-in triangular stainless steel packed tower (wherein the mass content of guanidine is 50%, the amount of substance of guanidine is 1mol) and Cyclohexylamine 100g (1mol), reacted at 65~70°C for 2h, then under vacuum at 200mmHg, rectified under reduced pressure to remove the ethanol generated by the reaction, took samples for analysis during the rectification process, and stopped the rectification after all the guanidines had been converted. The toluene solution of the intermediate compound guanidinourea was obtained.
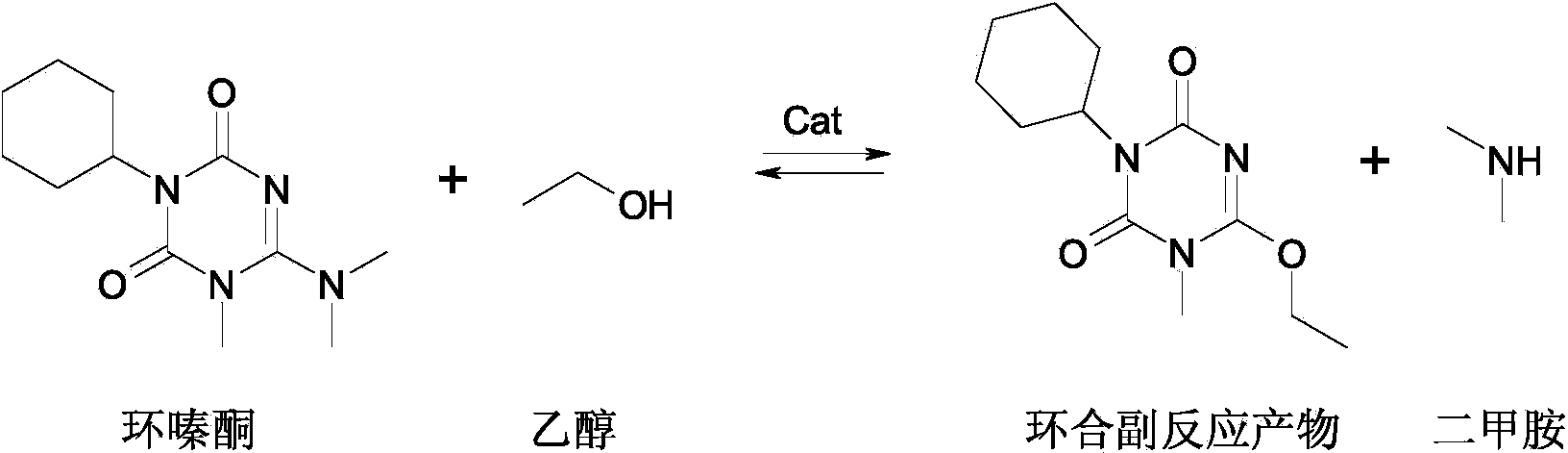
[0030] Add 1800g of toluene and the guanidinourea toluene solution obtained by the above reaction in a 5000mL reaction flask equipped with a mechanical stirrer, a thermometer, a dro...
Embodiment 2
[0032] In the reactant guanidine used in this embodiment, R=ethyl, and the molecular weight is 173.
[0033] Mechanical stirring, thermometer and internal diameter 15mm are housed, and in the 2000mL reaction bottle of length 300mm built-in triangular stainless steel packing tower, add the benzene solution 346g of 800g benzene, guanidine (wherein the mass content of guanidine is 50%, the amount of substance of guanidine is 1mol) and Cyclohexylamine 102g (1.02mol), react at 70-80°C for 3h, then under vacuum at 200mmHg, rectify under reduced pressure to remove the ethanol generated by the reaction, take samples for analysis during the rectification process, and stop the rectification after all guanidines have been converted , to obtain the benzene thin solution of the intermediate compound guanidyl urea.
[0034] Add 1800g of benzene and the guanidinourea benzene solution obtained by the above reaction in a 5000mL reaction flask equipped with a mechanical stirrer, a thermometer, ...
Embodiment 3
[0036] In the reactant guanidine used in this embodiment, R=ethyl, and the molecular weight is 173.
[0037] Mechanical stirring, thermometer and internal diameter 15mm are housed, add the chloroform solution 346g of 1400g chloroform, guanidine in the 2000mL reaction bottle of length 300mm built-in triangular stainless steel packed tower (wherein the mass content 50% of guanidine, the amount of substance of guanidine is 1mol) and Cyclohexylamine 105g (1.05mol), react at 55-60°C for 4h, then azeotropic distillation to remove the ethanol generated by the reaction, sampling and analysis during the rectification process, stop the rectification after all the guanidines have been converted to obtain the intermediate compound Guanidylurea dilute solution in chloroform.
[0038] Add 3000g chloroform and the dilute guanidinourea chloroform solution obtained by the above reaction in a 5000mL reaction flask equipped with a mechanical stirrer, a thermometer, a dropping funnel, a clear tub...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com