A vaccine that suppresses streptococcal bacteria and/or prevents streptococcal infections
A type A streptococcus and vaccine technology, which is applied in the direction of antibacterial drugs, bacterial antigen components, medical preparations containing active ingredients, etc., can solve problems that hinder the development of streptococci vaccines, achieve rapid elimination of pathogenic bacteria, and be easy to promote Effect of using and preventing the colonization of pathogenic bacteria
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
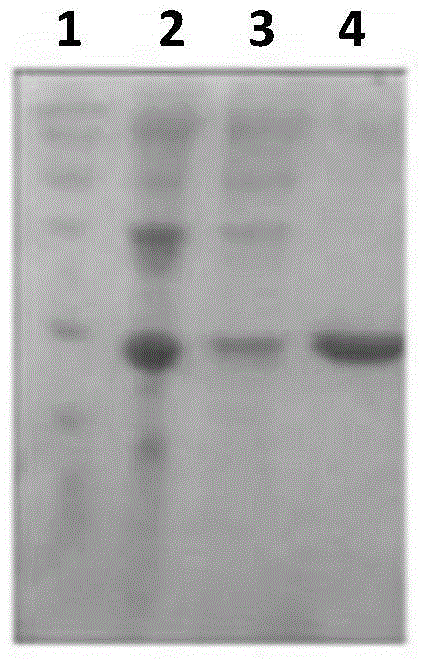
[0042] Embodiment 1, the preparation of sortase A
[0043] 1. Using the genomic DNA of type A Streptococcus M1 as a template, perform PCR amplification with a primer pair composed of F1 and R1 to obtain a PCR amplification product. The agarose gel electrophoresis of the PCR amplification product is shown in figure 1 .
[0044] F1: 5'-CTTA CATATG GTCTTGCAAGCACAAATGG-3';
[0045] R1: 5'-ATGTT CTCGAG CTAGGTAGATACTTGGTTATAAGA-3'.
[0046] 2. Digest the PCR amplification product of step 1 with restriction endonucleases NdeI and XhoI, and recover the digested product.
[0047] 3. Digest the vector pET28a(+) with restriction endonucleases NdeI and XhoI to recover a vector backbone of about 6000 bp.
[0048] 4. Ligate the digested product of step 2 with the vector backbone of step 3 to obtain the recombinant plasmid pET28a-SrtA. According to the sequencing results, the results of the recombinant plasmid pET28a-SrtA are described as follows: between the NdeI and XhoI restricti...
Embodiment 2
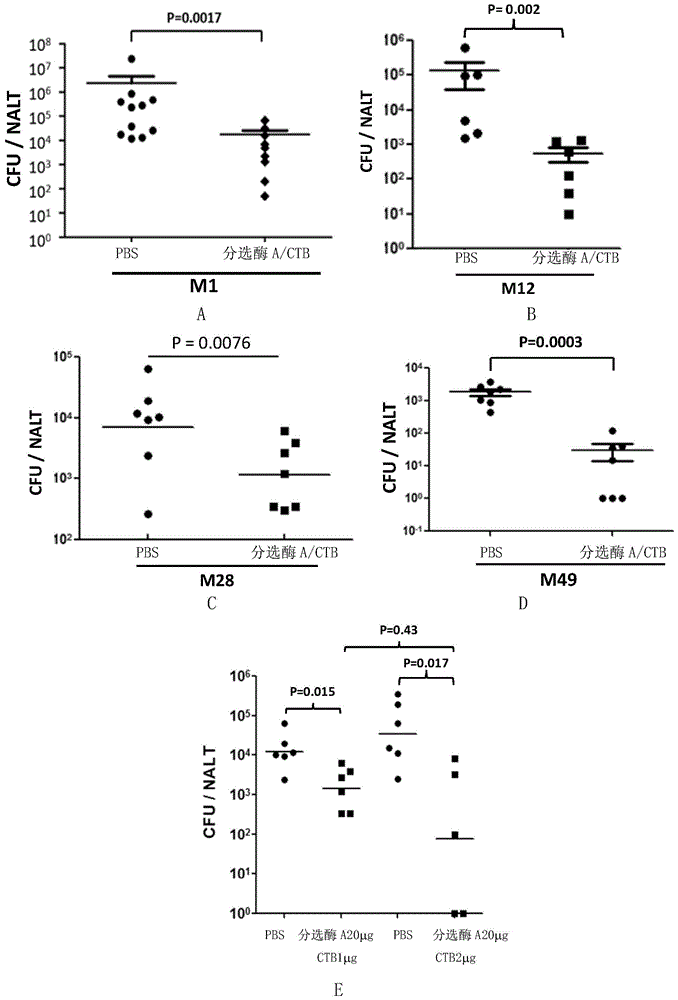
[0055] Example 2, Immune protection against type A streptococcal infection after nasal cavity inhalation of sortase A and CTB protein
[0056] 1. Female BALB / c mice aged 4-6 weeks were randomly divided into two groups, and the groups were treated as follows:
[0057] PBS group: on the 1st day, 7th day and 14th day of the experiment, PBS buffer was instilled through the nasal cavity;
[0058] Sortase A / CTB group: On the 1st day, the 7th day and the 14th day of the experiment, the vaccine solution was instilled through the nasal cavity respectively (the vaccine solution was obtained by mixing the sortase A solution prepared in Example 1 and the CTB protein solution, Each mouse was given 20 μg sortase A and 1 μg CTB protein each time).
[0059] On the 21st day of the experiment, the mice were challenged with live bacteria (type A Streptococcus M1, Type A Streptococcus M12, Type A Streptococcus M28 or Type A Streptococcus M49) by intranasal instillation (bacterial solution The c...
Embodiment 3
[0067] Example 3. Mucosal immunization with sortase A and CTB protein induces an immune response dominated by Th17 cells
[0068] Th17 cells play a major role in mucosal immune protection. The purpose of this example is to verify the effect of sortase A and CTB protein on Th17 cell activation.
[0069] Female BALB / c mice aged 4-6 weeks were randomly divided into four groups, and the grouping was handled as follows:
[0070] PBS group: on the 1st day, 7th day and 14th day of the experiment, PBS buffer was instilled through the nasal cavity;
[0071] Sortase A group: On the first day, the seventh day and the 14th day of the experiment, the sortase A solution prepared in Example 1 was instilled through the nasal cavity respectively (each mouse was given 20 μg sortase A each time);
[0072] CTB group: On the first day, the seventh day and the 14th day of the experiment, the CTB protein solution was instilled through the nasal cavity respectively (each mouse was given 1 μg CTB pro...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com