Famciclovir direct compressed tablet and preparation method thereof
A famciclovir, direct compression tablet technology, applied in the field of medicine, can solve the problems such as the improvement of quality controllability and product stability that are not mentioned, and achieve the advantages of improving fluidity and compressibility, simplifying process and improving stability. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] Embodiment 1: the selection of famciclovir bulk drug
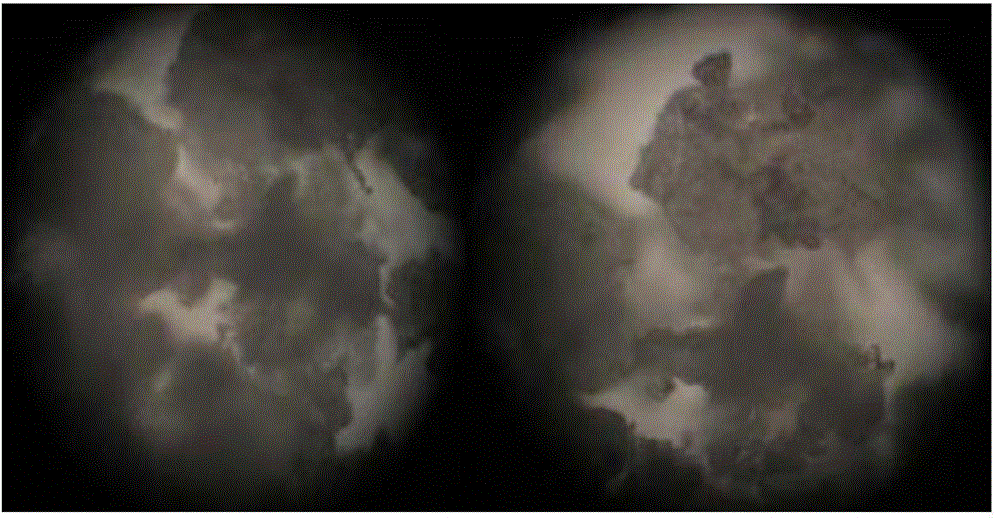
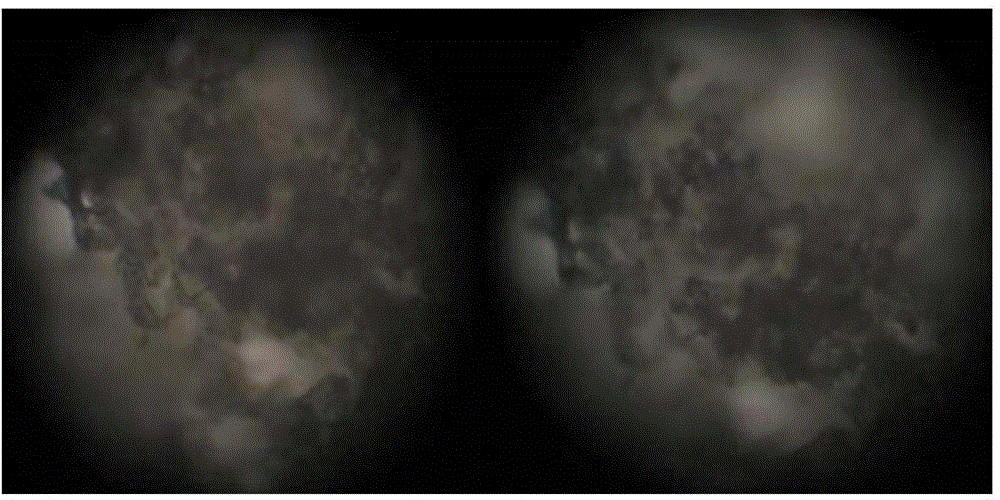
[0043] Famciclovir has good water solubility and accounts for a large amount of prescriptions. Therefore, it is necessary to investigate the particle shape and particle size of the raw material drug to ensure that the raw material drug has good fluidity and compressibility, and is suitable for the direct compression process. The famciclovir APIs produced by Zhejiang Chetou Pharmaceutical Co., Ltd. and Changzhou Kangli Pharmaceutical Co., Ltd. were compared.
[0044] Observe under a microscope at 400 times magnification, such as figure 1 As shown, the particle size of famciclovir API produced by Zhejiang Chetou Pharmaceutical Co., Ltd. is relatively large, the shape is close to spherical, and there are no needle-like and flake-like particles. Such as figure 2 As shown, the famciclovir API produced by Changzhou Kangli Pharmaceutical Co., Ltd. has a smaller particle size and more slender rod-shaped particles. As de...
Embodiment 2
[0047] Embodiment 2: the investigation of direct compression technology feasibility
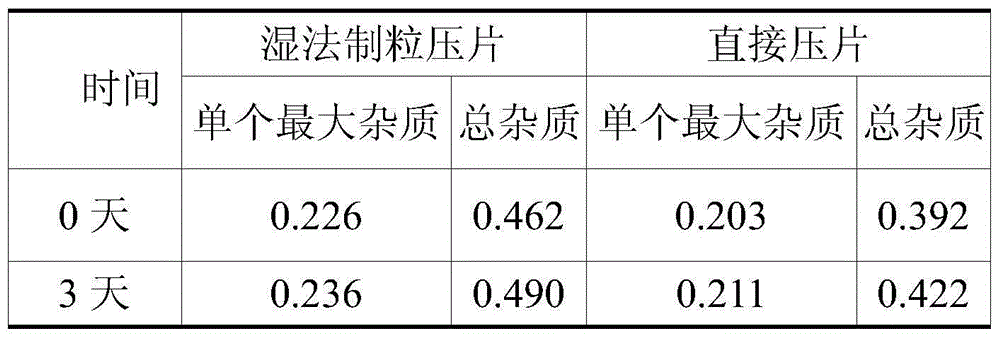
[0048] Famciclovir tablets were prepared by powder direct compression and wet granulation techniques respectively, and the prescriptions are shown in Table 1:
[0049] Table 1 Famciclovir tablet process screening prescription (every 1000 contains, g)
[0050] Raw materials
Prescription 1 / Direct Compression
Recipe 1 / wet granulation
famciclovir
125.0
125.0
29.0
29.0
3.0
3.0
2.0
2.0
1.0
1.0
[0051] Preparation Process
[0052] (1) Direct tableting process
[0053] Mix famciclovir, lactose, hydroxypropyl cellulose and sodium carboxymethyl starch evenly, finally add magnesium stearate, mix evenly and press into tablets (DPG-30 single-punch tablet press, Beijing Sinopharm Longli Technology Co., Ltd., manual tablet press) .
[0...
Embodiment 3
[0077] According to the following prescription, the direct compression method is used to make tablets, and the prescription is shown in Table 3.
[0078] Table 3 Famciclovir tablet process screening prescription (every 1000 contains, g)
[0079] Raw materials
[0080] Preparation Process:
[0081] 1) Famciclovir and lactose (Flowlac100) pass through a 26-mesh sieve and mix well
[0082] 2) Mix colloidal silicon dioxide and microcrystalline cellulose (102), pass through a 26-mesh sieve, and mix with the mixture obtained in 1)
[0083] 3) Add the prescribed amount of magnesium stearate to the mixture obtained in 2) and mix evenly
[0084] 4) Tablet pressing (DPG-30 single-punch tablet press, Beijing Sinopharm Longli Technology Co., Ltd.).
[0085] The results are shown in Table 4.
[0086] Table 4 Famciclovir Tablets Prescription Screening Results
[0087] investigation
[0088] *Measurement method of angle of repose: fixed funnel method to measure the...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
| Medium volume | aaaaa | aaaaa |
| Hardness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com