Polypeptide, pharmaceutical composition and preparation method of pharmaceutical composition
A technology of pharmaceutical compounds and compositions, applied in the field of polypeptides and a pharmaceutical composition and its preparation, can solve problems such as good drug loading effect, and achieve good drug loading effect, good stability, and good biocompatibility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
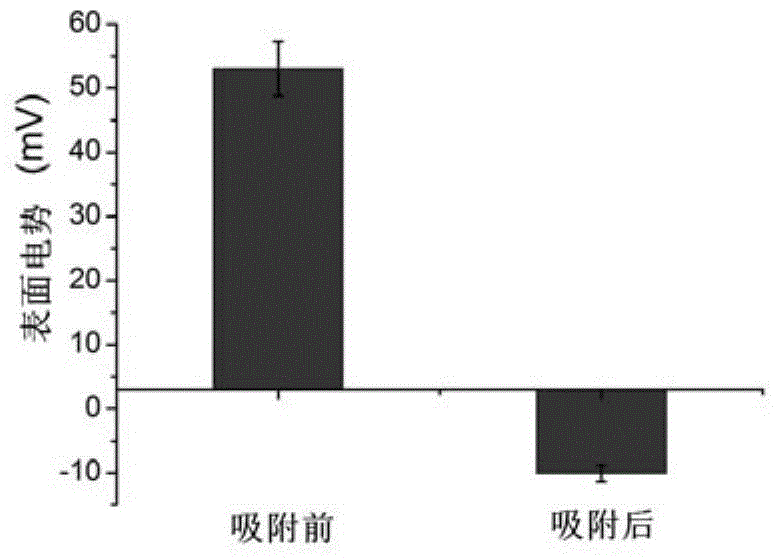
[0030] The present invention also provides a preparation method of a pharmaceutical composition, which comprises contacting a solution of a pharmaceutical compound with the polypeptide represented by the above-mentioned formula (1) of the present invention to obtain a contacted material.
[0031] In the present invention, there is no particular limitation on the above-mentioned contacting method, and it can be various methods routinely used in the art to prepare nano-pharmaceutical compositions, as long as the drug compound solution can be combined with the polypeptide represented by formula (1) It is enough to form a pharmaceutical composition. Preferably, the above-mentioned conditions for contacting the drug compound solution with the polypeptide represented by the formula (1) of the present invention may include: a pH value of 6-8, a temperature of 4-45°C, and a time of 12 -48 hours. Preferably, the contacting conditions include: a pH value of 6.5-7.5, a temperature of 20...
Embodiment approach
[0032] According to a preferred embodiment of the present invention, the preparation method of the pharmaceutical composition comprises mixing the pharmaceutical compound solution with the polypeptide represented by the formula (1) of the present invention, and then dialyzing with a dialysis bag without being coated Buried drug compounds are eliminated during this process, thereby obtaining a pharmaceutical composition. As the dialysis bag, for example, a dialysis bag with a molecular weight cut off of 1 kDa can be used. The dialysis is preferably performed in a phosphate buffer (eg, pH value is 7.4, 150 mmol / L), more preferably the phosphate buffer is changed every 3-5 hours, and the dialysis is performed for 20-30 hours.
[0033] According to a preparation method of a pharmaceutical composition provided by the present invention, the dosage of the pharmaceutical compound is 0.05-1 part by weight relative to the polypeptide represented by the above formula (1). Preferably, th...
preparation example 1
[0050] This preparation example is used to synthesize the polypeptide provided by the present invention.
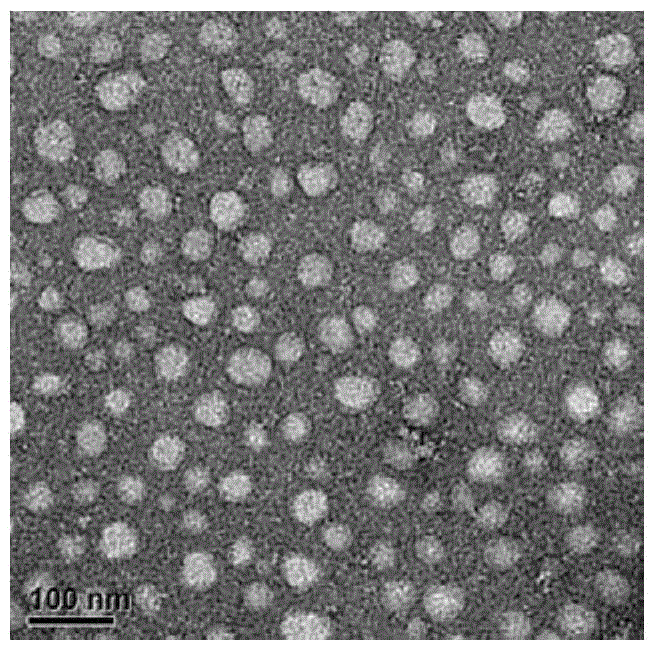
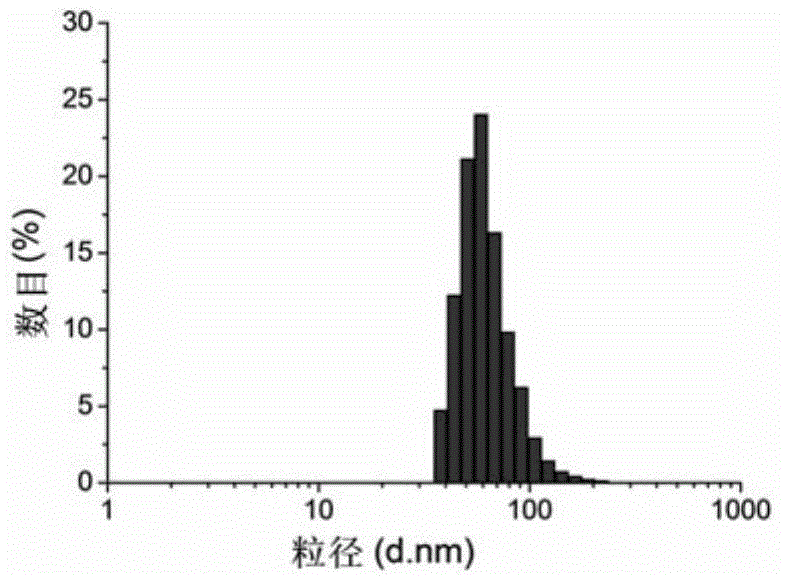
[0051] According to the method provided in the literature (Lihong Liu et al., Nature nanotechnology, 2009, Vol4, July), synthesize the polypeptide shown in formula (1), the difference is that m of the polypeptide synthesized in this preparation example is 1, and n is 9. The C-terminal amino acid of the polypeptide is arginine, and the polypeptide sequence does not contain tyrosine. Identified by mass spectrometry (time-of-flight mass spectrometry, MALDI-TOF, BRUKER, microflex.LRF, USA), its molecular weight is 1878Da, which is completely consistent with the molecular weight of the polypeptide (m is 1, n is 9) shown in formula (1), named The polypeptide represented by the formula (1) (m is 1, n is 9) is AP.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com