Cyclic polycaprolactone-polyethylene glycol amphipathic block copolymer and preparation and applications
An amphiphilic block and polycaprolactone technology is applied in the directions of non-active ingredients such as medical preparations and pharmaceutical formulations, which can solve the problem of the absence of amphiphilic block copolymers, and achieve low cytotoxicity and good The effect of drug-carrying capacity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0047] Example 1 Preparation of acetylenic anhydride
[0048] In the following examples of the present invention, the synthetic route of the acetylenic anhydride used is as follows:
[0049]
[0050] The specific operations are as follows:
[0051] 9.3 g (93.0 mmol) of succinic anhydride and 2.6 g (21.4 mmol) of 4-dimethylaminopyridine (DMAP) were dissolved in 20 mL of dry dichloromethane (DCM). 6.0g (107.0mmol) of propynol was dissolved in 10mL of dry DCM, slowly added dropwise to the above suspension solution, protected by argon, and stirred at 25°C for 36h. After the reaction, add 50mL DCM to dilute, 10% NaHSO 4 Wash three times with aqueous solution (3×20mL), 10% NaHCO 3 Wash with aqueous solution three times (3×20 mL). Use anhydrous Na for organic phase 2 SO 4 Drying, filtering with suction, and rotary evaporation to obtain a white solid powder (Compound 1, 10.0 g in the above figure, yield: 68.9%).
[0052] 8.0 g (51.3 mmol) of compound 1 was dissolved in 40 mL of dry DCM, and...
Embodiment 2
[0053] Example 2 Preparation of small molecules terminated with azide groups at both ends (hereinafter referred to as double-headed azide-terminated small molecules)
[0054] In the following embodiments of the present invention, the synthetic route of the double-headed azide-terminated small molecule used is as follows:
[0055]
[0056] The specific operations are as follows:
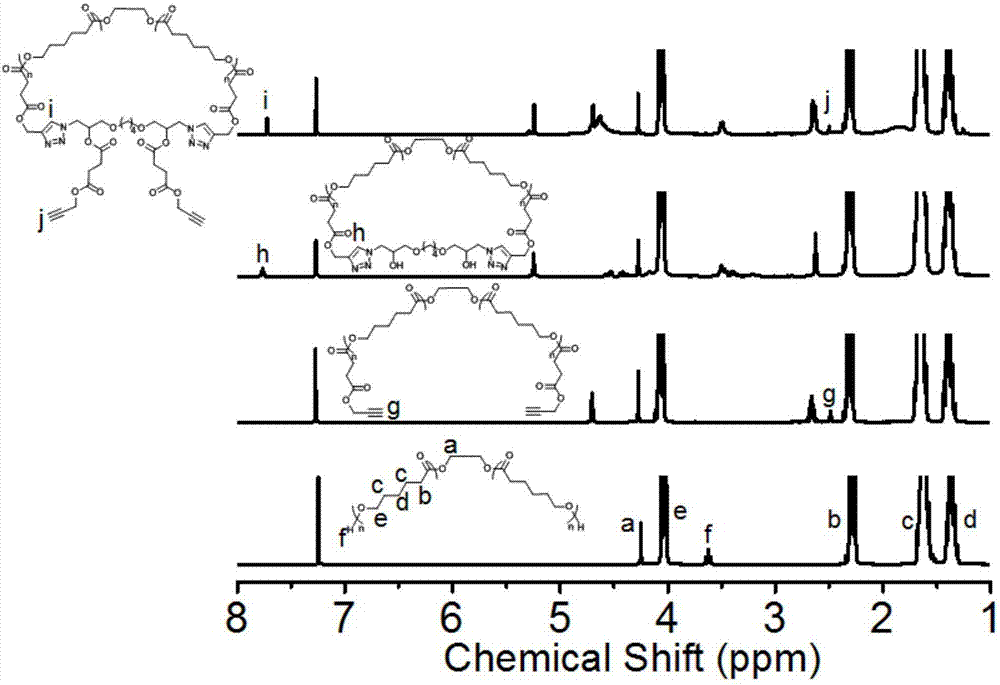
[0057] Mix 3.0g (14.8mmol) 1,4-butanediol diglycidyl ether, 4.8g (78.0mmol) NaN 3 And 4g (74.0mmol) NH 4 Cl was added to 30 mL of DMF solvent, and the reaction was stirred at 50°C for 24 h. After the reaction is over, cool to room temperature, add 300 mL DCM for dilution, and wash with water to remove DMF. Use anhydrous Na for the DCM phase 2 SO 4 Drying, suction filtration, and rotary evaporation to obtain a white waxy solid, which is a double-headed azide-terminated small molecule (compound 3 in the above figure, 3.7 g, yield: 86.4%). NMR characterization: 1 H NMR(300MHz, DMSO-d 6 ,δ,ppm):5.22(d,2H),3.77...
Embodiment 3
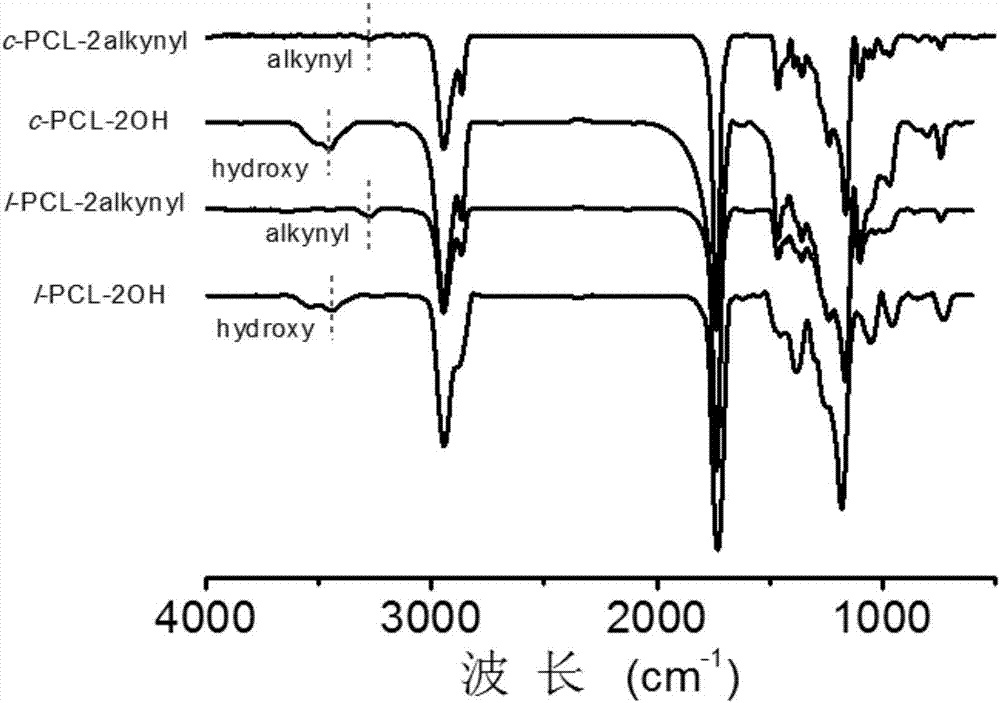
[0058] Preparation of Example 3c-PCL-2alkynyl
[0059] The synthesis steps are as follows:
[0060] (1) Synthesis of l-PCL-2OH
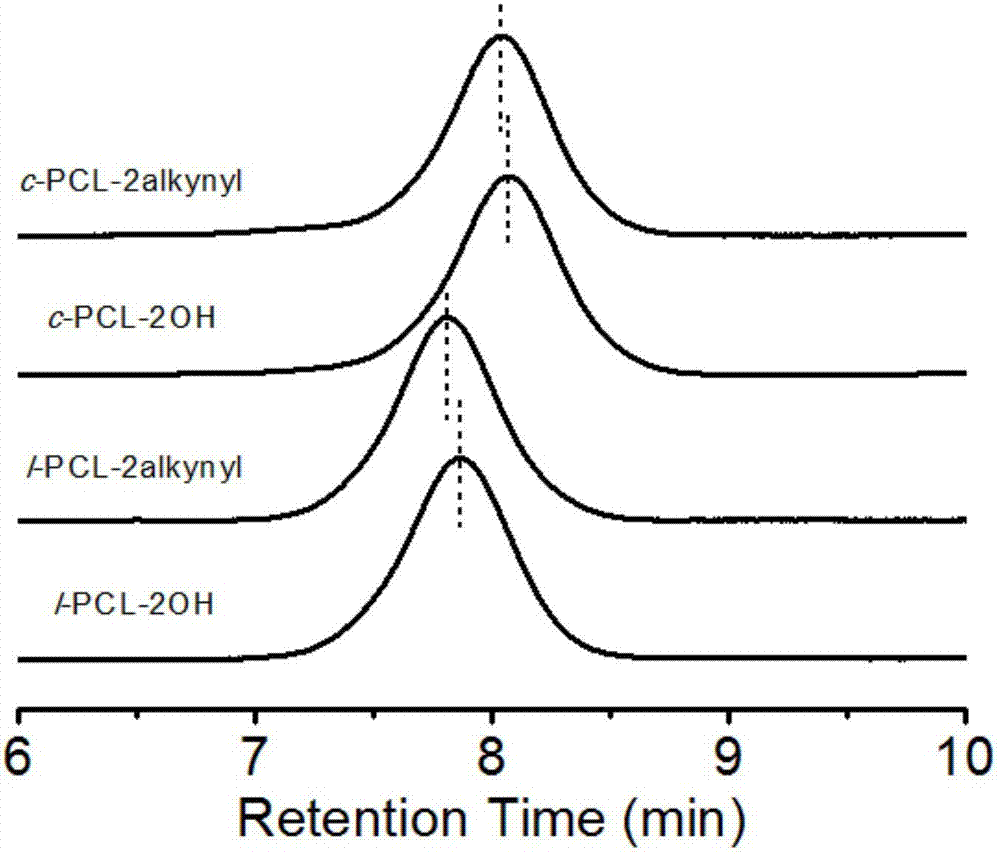
[0061] Add a stir bar to a 5 mL ampoule, apply vacuum heating on the double-row tube, fill it with argon and cool it three times, and put it in the glove box. Add 4.0 mL (36.0 mmol) ε-CL (ε-caprolactone) and 40.0 μL (0.7 mmol) of initiator ethylene glycol into the ampoule. Then add 80.0μL (0.8mol / L) catalyst Sn(Oct) 2 In toluene. Seal with a rubber tube, stir and react at 100°C for 2h. After the reaction is over, take it out of the glove box, cool it immediately, add THF to dissolve it, precipitate with ice anhydrous ether, filter with suction, and dry the filter cake in a vacuum oven at 25°C overnight to obtain a white powder, which is l-PCL-2OH (2.9g , Yield: 70.4%). NMR spectrum ( 1 H NMR) measurement result: M n,NMR =4900g / mol; SEC measurement result: M n,SEC =8300g / mol,
[0062] (2) Synthesis of l-PCL-2alkynyl
[0063] Add 0.5g (0.1mmol) of l-PC...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com