Antitumor drug and preparation method thereof
An anti-tumor drug and drug technology, applied in the field of drugs, can solve the problems of poor efficiency, small toxic and side effects, and high-efficiency nanovesicles, and achieve the effects of efficient loading, avoiding losses and toxic side effects, and promoting cell proliferation.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
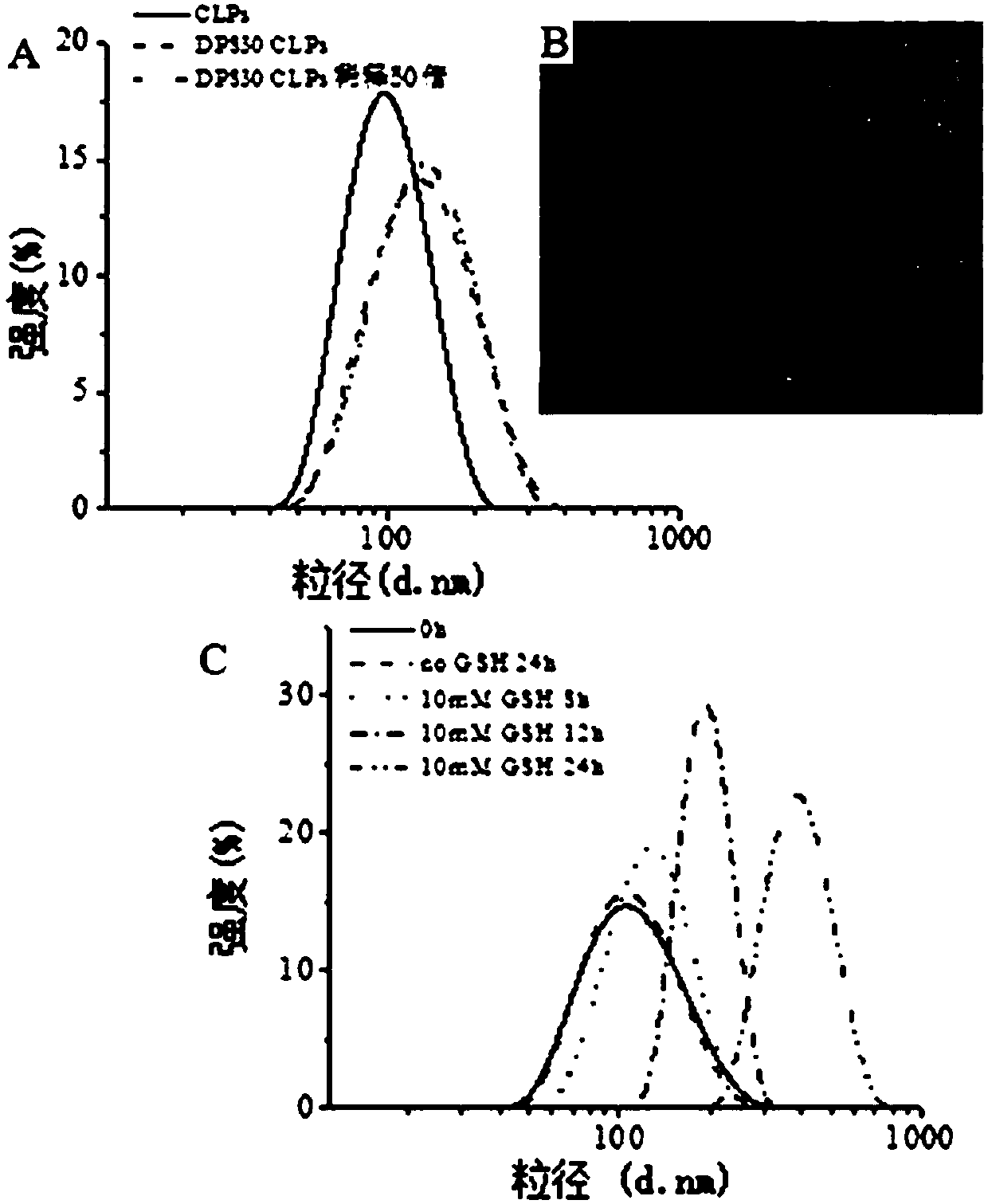
Image
Examples
Embodiment 1
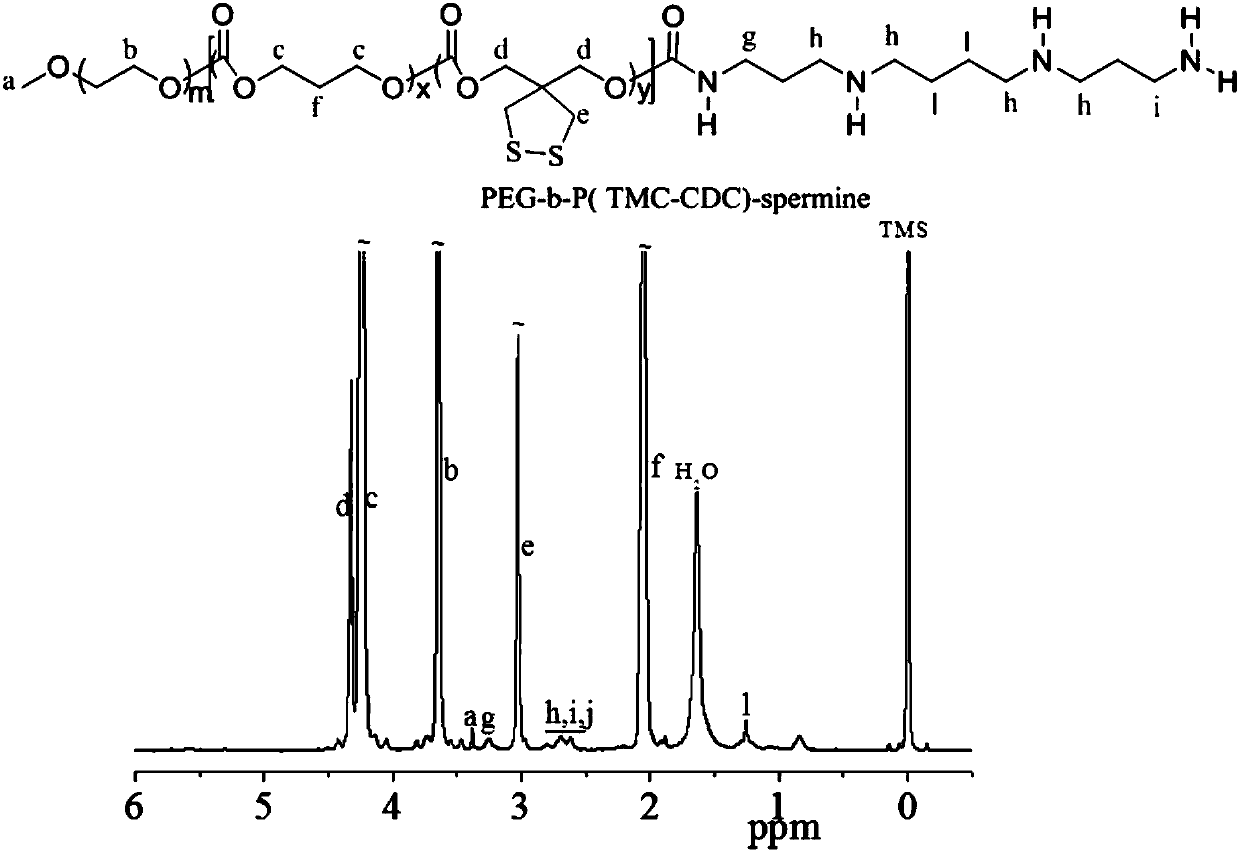
[0078] Example 1 Synthetic polymer PEG5k-P(DTC4.6k-TMC13.5k)-SP
[0079] The synthesis is divided into two steps. First, ring-opening polymerization is used to prepare PEG5k-P (DTC4.6k-TMC13.5k) diblock copolymer. The specific operation is as follows. Under nitrogen environment, MeO-PEG-OH ( M n = 5.0 kg / mol, 0.20 g, 40 μmol), TMC (0.6 g, 5.9 mmol) and DTC (0.192g, 1.0 mmol) were dissolved in dichloromethane (DCM, 6.8 mL), and the ring-opening polymerization catalyst was added rapidly, Such as zinc bis(bistrimethylsilyl)amine (7.7 mg, 20 μmol). The airtight reactor was sealed and placed under magnetic stirring in an oil bath at 40 °C for 24 hours. After terminating the reaction with glacial acetic acid, precipitate twice in glacial ether, filter with suction, and dry under vacuum at room temperature to obtain the product. Yield: 90.3%. 1 H NMR (400 MHz, DTCl 3 ): PEG: δ 3.38,3.65; TMC: δ 4.24, 2.05; DTC: δ 4.32, 3.02. According to NMR calculation, the molecular weight of...
Embodiment 2
[0083] Example 2 Synthesis of block copolymer Mal-PEG6k-P (DTC4.8k-TMC15.2k)-SP
[0084] The synthesis of Mal-PEG6k-P (DTC4.8k-TMC15.2k)-SP is similar to that of Example 1, and is also divided into two steps, except that the initiator MeO-PEG-OH in the first step is replaced by Malay Imide-functionalized Mal-PEG6k-OH, ring-opening polymerization of TMC and DTC to obtain Mal-PEG6k-P (DTC4.8k-TMC15.2k), then its terminal hydroxyl was activated with NPC, and then reacted with the primary amine of spermine be made of. The specific operation is similar to the first embodiment. Yield: 90.2%. 1H NMR (400 MHz, DTCl3): PEG: δ3.38, 3.65; TMC: δ 4.24, 2.05; DTC: δ 4.32, 3.02, and the characteristic peaks of Mal and spermine. The number-average molecular weight of the polymer was calculated as 6.0-(4.8-15.2)-0.2 kg / mol through the integral ratio of the characteristic peak area.
Embodiment 3
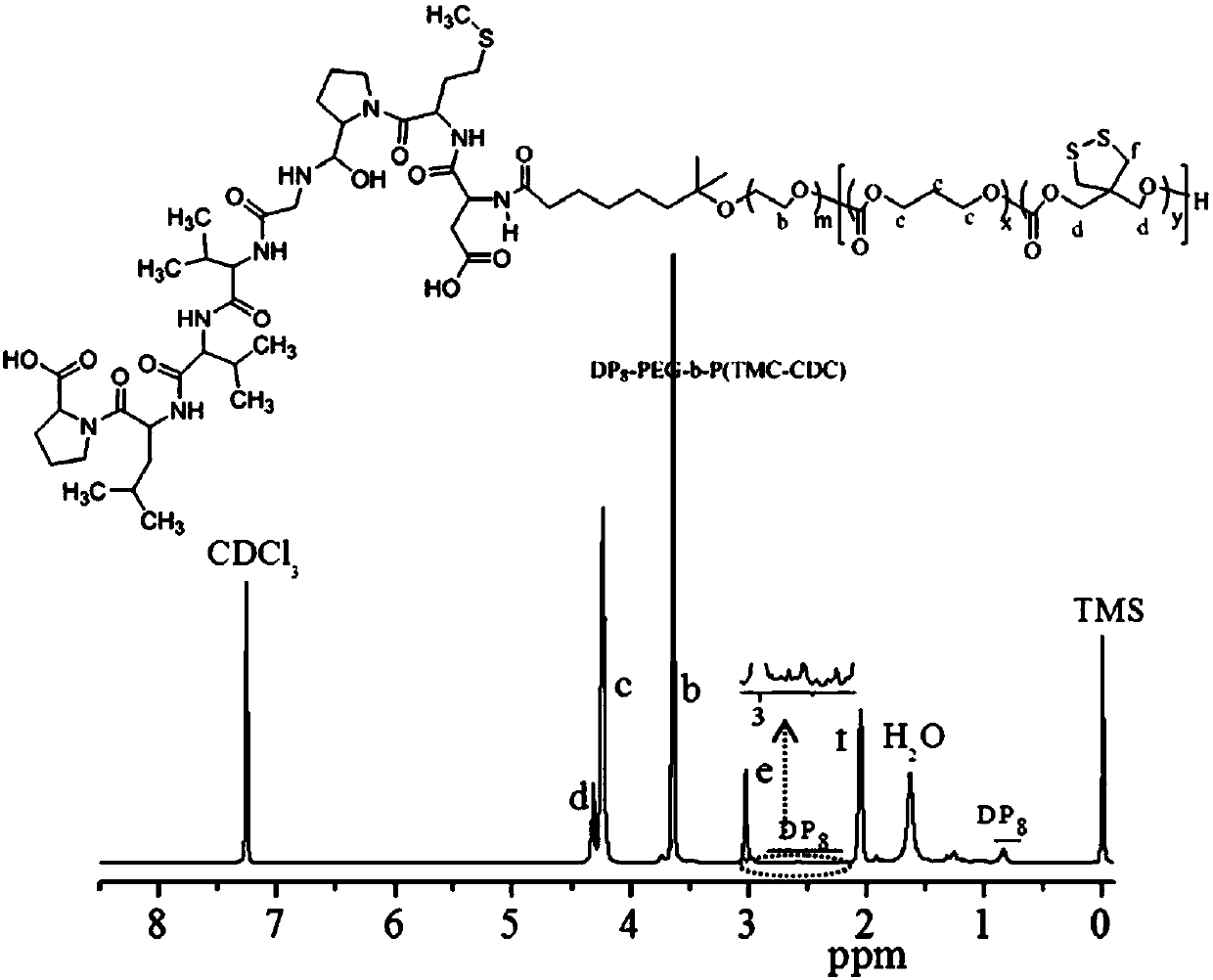
[0085] Example 3 Synthesis of Targeted Diblock Polymer DP8-PEG6.5k-P(DTC6k-TMC15k)
[0086] The synthesis of DP8-PEG6.5k-P(DTC6k-TMC15k) is similar to that of Example 1, which is also divided into two steps. In the first step, the initiator MeO-PEG-OH in the first step of Example 1 is replaced by NHS-PEG6.5k-OH functionalized with N-hydroxysuccinimide, and ring-opening polymerization of TMC and DTC is carried out to obtain NHS-PEG6 .5k-P(DTC6k-TMC15k), that is, add 0.15 g ( 0.781 mmol) DTC, 0.43 g of TMC, 0.2 g ( 0.0154 mmol) NHS-PEG6.5k-OH and 5 ml DCM was dissolved, and then 5.9 mg (0.0154 mmol) of a ring-opening polymerization catalyst such as bis(bistrimethylsilyl)amine zinc was added, and the subsequent treatment was the same as in Example 1. NMR calculated NHS-PEG6.5k-P (DTC6k-TMC15k) by integral area. In the second step, the polypeptide DMAPTVLP (DP8) is added according to the molar ratio of its amino group to NHS-PEG6.5k-P (DTC6k-TMC15k) 3:1, amidation reaction at 30...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight distribution | aaaaa | aaaaa |
| size | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com