Faropenem sodiumcomposition for direct tabletcompression and preparation method of faropenem sodiumcomposition
A technology of faropenem sodium and its composition, which is applied in the field of faropenem sodium composition and its preparation, can solve problems such as poor stability, and achieve the effects of improving production efficiency, saving man-hours, and improving product stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
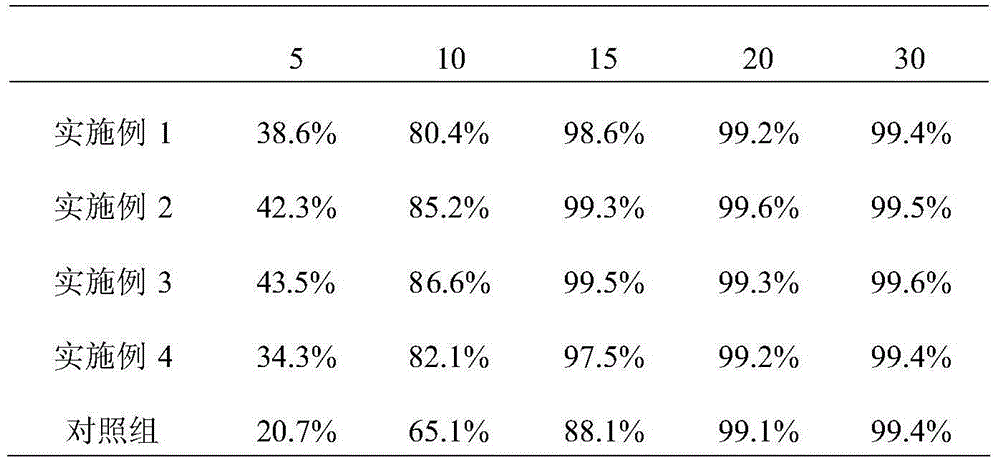
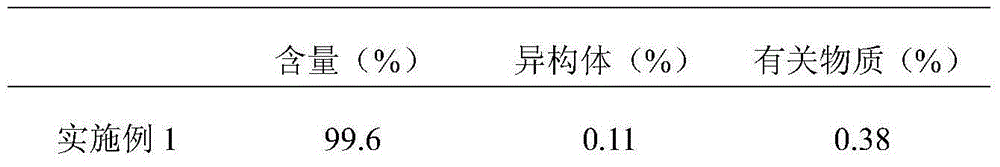
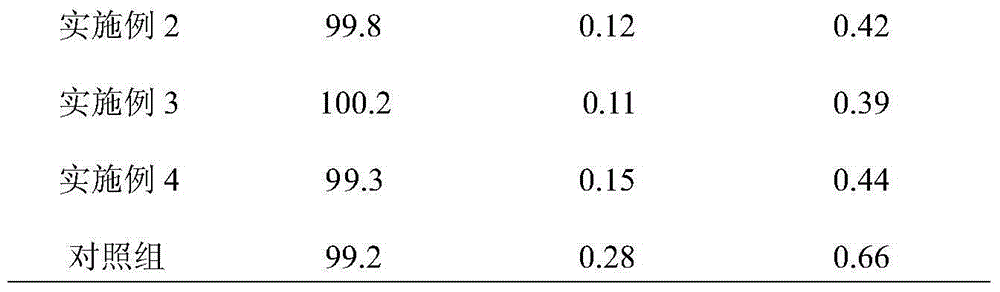
Embodiment 1
[0015] 100g of faropenem sodium (calculated as faropenem) with an average particle size of 150μm-160μm, 50g of microcrystalline cellulose 112, 30g of pregelatinized starch, 20g of hydroxypropyl cellulose, 1g of pharmaceutical micropowder silica gel, and 1g of magnesium stearate. Into 1000 pieces.
[0016] Preparation method: Weigh faropenem sodium, microcrystalline cellulose 112, pregelatinized starch and hydroxypropyl cellulose according to the prescription amount, and mix them evenly. Then add medicinal micropowder silica gel and magnesium stearate, mix evenly, compress into tablets, and coat with a film to obtain the product.
Embodiment 2
[0018] 100g faropenem sodium with an average particle size of 180μm-190μm (calculated as faropenem), 60g anhydrous lactose, 40g pregelatinized starch, 30g calcium hydrogen phosphate, 1g pharmaceutical micropowder silica gel, 1g magnesium stearate, 3g talc powder, Made in 1000 pieces.
[0019] Preparation method: Weigh faropenem sodium, anhydrous lactose and pregelatinized starch according to the prescription amount, and mix them evenly. Then add medicinal micropowder silica gel, magnesium stearate and talcum powder, mix evenly, press into tablets, and coat with a film to obtain the product.
Embodiment 3
[0021] 100g of faropenem sodium (calculated as faropenem) with an average particle size of 180μm-190μm, 60g of anhydrous lactose, 40g of pregelatinized starch, 30g of calcium hydrogen phosphate, 1g of pharmaceutical micropowder silica gel, 1g of weight average molecular weight Mn=2.717×10 5 And number average molecular weight Mw=1.232×10 5 Seed melon polysaccharide sulfate, 1g magnesium stearate, 3g talc powder, made into 1000 tablets.
[0022] Preparation method: Weigh faropenem sodium, anhydrous lactose and pregelatinized starch according to the prescription amount, and mix them evenly. Then add medicinal micropowder silica gel, seed melon polysaccharide sulfate, magnesium stearate and talcum powder, mix evenly, press into tablets, and coat with film to obtain the product.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Average particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com