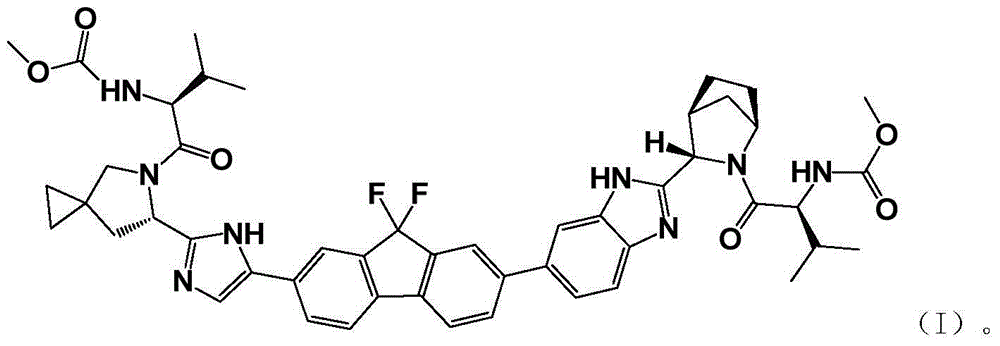
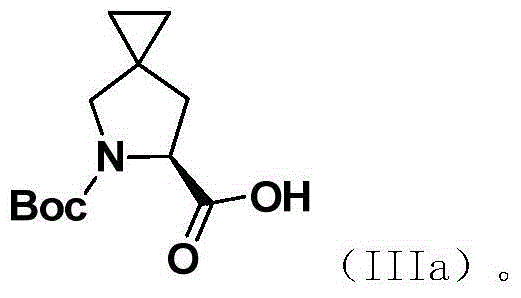
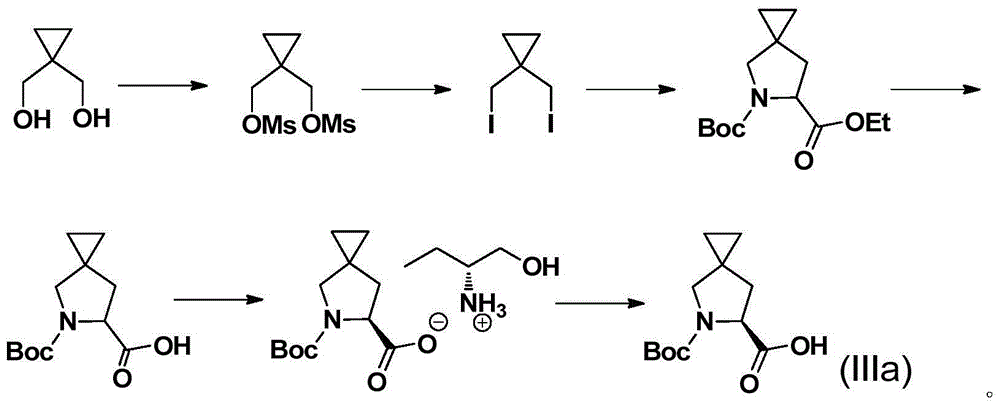
Preparation method of (S)-5-R-5-azaspiro (2,4) heptane-6-carboxylic acid
A technology of -5-R-5-, -5-boc-5-, applied in the field of medicinal chemistry, can solve the problems of reducing the total yield and achieve the effect of increasing the yield and reducing the cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0051] Embodiment 1 prepares compound 3
[0052]
[0053] At 0°C, compound 2a (22.3 g, 105 mmol) and 2 (31.5 g, 100 mmol) in DMAc (40 ml) were added dropwise to NaH (12 g, 300 mmol) in DMAc (110 ml). After dropping, the reaction solution was stirred at this temperature for 6 hours. After HPLC showed that less than 0.5% of compound 2 remained, 230 ml of 2N HCl was added dropwise at the same temperature. After the drop was completed, the temperature was raised to room temperature, and after stirring for 4 hours, 300 ml of ethyl acetate was added to the reaction solution for liquid separation. Saturated Na 2 CO 3 The pH of the solution was adjusted to alkaline. 300 ml of n-heptane was added thereto, and the liquid was separated. The aqueous phase was extracted with 200ml of n-heptane, the organic phases were combined, dried, and concentrated under reduced pressure to obtain 13g of oily substance, yield: 75%, enantiomeric excess (ee) was 99.2%.
[0054] 1 H NMR (400MHz, C...
Embodiment 2
[0055] Embodiment 2 prepares compound 4
[0056]
[0057] At 0°C, potassium carbonate powder (20.7g, 150mmol) was added to compound 3 (13g, 75mmol) in DCM (100ml), and after stirring at this temperature for 30min, di-tert-butyl dicarbonate (35ml, 150mmol) was added dropwise. After the drop, the temperature was raised to room temperature and stirred for 2 hours. After HPLC showed that the remaining compound 3 was less than 0.5%, 100ml of water was added to separate the organic phase. The organic phase was washed twice with 70ml of saturated saline, dried over anhydrous sodium sulfate, and spin-dried to obtain Light gray solid 19.3g, yield: 94%.
[0058] 1 H NMR (400MHz, CDCl 3 ):δ8.10(s,1H),4.09(t,4.8Hz,1H),3.68(s,3H),3.54(d,J=6.8Hz,1H),3.23(d,J=6.8Hz,1H ),2.03(dd,J=8.4Hz,4.8Hz,1H),1.92(d,J=8.4Hz,4.8Hz,1H),1.42(s,9H),0.55(m,2H),0.45(m, 2H) ppm.
Embodiment 3
[0059] The preparation of embodiment 3 compound 5
[0060]
[0061] At 0°C, PBr was slowly added dropwise to a solution of compound 4 (19.1g, 70mmol) in 60ml of dichloromethane (150ml) 3 (37.8g, 140mmol), after dropping, stirred overnight at room temperature. Wash with saturated potassium carbonate solution (100ml) and dry over anhydrous sodium sulfate to obtain 22.5g of light yellow solid, yield: 97%.
[0062] 1 H NMR (400MHz, CDCl 3 ):δ8.10(s,1H),4.09(t,4.8Hz,1H),3.68(s,3H),3.24(d,J=6.8Hz,1H),2.94(d,J=6.8Hz,1H ),2.03(dd,J=8.4Hz,4.8Hz,1H),1.92(d,J=8.4Hz,4.8Hz,1H),1.42(s,9H),0.55(m,2H),0.45(m, 2H) ppm.
PUM
| Property | Measurement | Unit |
|---|---|---|
| purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com