A kind of synthetic method of 2,6-dichloropyridine
A technology for dichloropyridine and a synthesis method, applied in the field of chemistry, can solve problems such as unsatisfactory selectivity of 2,6-dichloropyridine, and achieve the effects of prolonged reaction time, reduced speed and low energy consumption
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] Add 227 grams of 2-chloropyridine (2.0 moles) in a four-mouth jacketed glass reactor equipped with a reflux condenser, an air guide tube, a thermowell and a mechanical stirrer. Heating to 160°C, under the irradiation of 40W ultraviolet lamp, passing chlorine gas at a rate of 0.22mol / h for about 10 hours under normal pressure to react,
[0023] In the meantime, by Petite The oil bath circulation system realizes the temperature program of the reactor, so that the internal temperature of the reaction bottle gradually rises to 190°C. After the introduction of chlorine gas, the reaction solution was cooled to room temperature under a nitrogen atmosphere, and samples were taken for quantitative analysis by HPLC (the analysis results are shown in Table 1).
Embodiment 2
[0025] The reaction conditions were exactly the same as in Example 1, except that a 40W blue light was used as the light source (reaction results are shown in Table 1).
Embodiment 3
[0027] The reaction conditions are exactly the same as in Example 1, except that a 40W fluorescent lamp is used as the light source (reaction results are shown in Table 1).
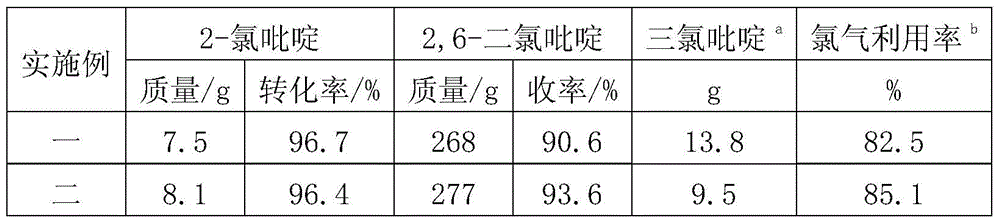
[0028] Table 1. Chlorination reaction results using different light sources
[0029]
[0030]
[0031] a Trichloropyridine is a mixture of 2,3,6-trichloropyridine and 2,4,6-trichloropyridine; b Chlorine gas utilization refers to the ratio of the amount of chlorine gas consumed to generate 2,6-dichloropyridine to the total amount of chlorine gas introduced
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com