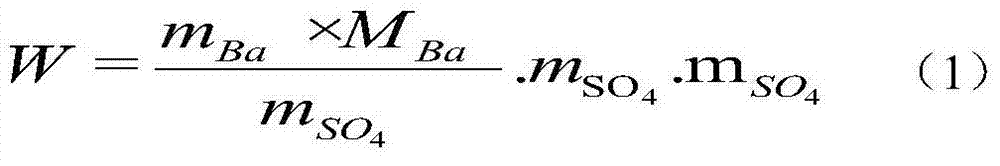Method for titration of sulphate radical
A sulfate and titration technology, applied in the field of analytical chemistry, can solve the problem that sulfate cannot be accurately titrated, and achieve the effect of a wide range of applications
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0062] Embodiment 1: Accurately weigh 5g of sample solution containing sulfate radicals in a conical flask, add 3mL of acetic acid solution (A), make the pH of the solution about 2.1, add about 5mL of absolute ethanol, mix well, add alizarin dropwise Red indicates 4 drops (alizarin red concentration corresponds to 0.000872%). Titrate with barium chloride solution to pink as the end point. The content of sulfate in the sample can be obtained according to the consumption of the titrant, and the content of sulfate is calculated according to the standard potassium sulfate, and the error of the two calculations is 0.25%. The experimental result data is calculated according to formula (1).
Embodiment 2
[0063] Embodiment 2: Accurately take by weighing 5g of the sample solution containing sulfate radicals in the Erlenmeyer flask, add 4mL of acetic acid solution (A), make the solution pH about 3.5, add about 6mL of absolute ethanol, add dropwise alizarin red after mixing 13 drops of indicator (alizarin red concentration is equivalent to 0.00263%). Titrate with barium chloride solution to pink as the end point. The content of sulfate radical in the sample can be obtained according to the consumption of the titrant, and the content of sulfate radical can be calculated according to the standard potassium sulfate at the same time, and the error between the two calculations is 0.18%. The experimental result data is calculated according to formula (1).
Embodiment 3
[0064] Embodiment 3: Accurately take by weighing 5g of the sample solution containing sulfate radical in the Erlenmeyer flask, add 5mL acetic acid solution (A), make the solution pH about 3.4, add about 5mL dehydrated alcohol, add dropwise alizarin red after mixing 11 drops of indicator (alizarin red concentration is equivalent to 0.00220%). Titrate with barium chloride solution to pink as the end point. The content of sulfate radical in the sample can be obtained according to the consumption of the titrant, and the content of sulfate radical can be calculated according to the standard potassium sulfate at the same time, and the error of the two calculations is 0.22%. The experimental result data is calculated according to formula (1).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com