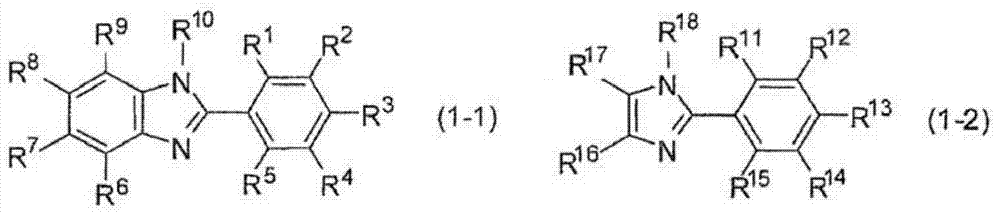
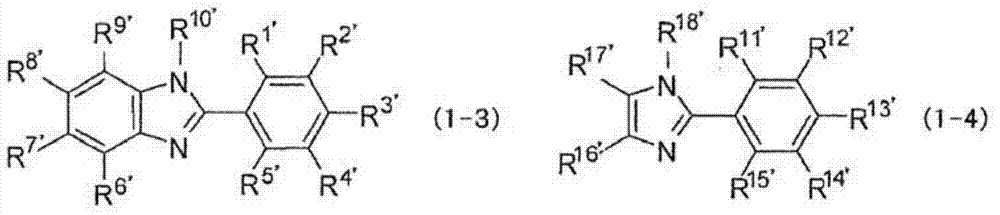
Hardenable composition, hardened film and forming method thereof, and compound
A composition and hardening technology, applied in chemical instruments and methods, compounds of group 4/14 elements of the periodic table, organic chemistry, etc., can solve the problem of insufficient solvent resistance and storage stability of the hardened film Excellent storage stability and low-temperature curability, excellent storage stability, and excellent low-temperature curability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0159]
[0160] The curable composition is prepared by uniformly mixing [A] compound and [B] polymerizable compound, preferably [C] alkali-soluble resin, [D] solvent, and other optional components. The curable composition is preferably used in a solution state by being dissolved in the [D] solvent, but the solvent may be omitted.
[0161] The solid content concentration (ratio of components other than the solvent in the composition solution) in the curable composition can be set according to the purpose of use, desired film thickness, and the like. The prepared solution of the curable composition may be used after being filtered using a millipore filter having a pore diameter of about 0.05 μm to 0.5 μm or the like.
[0162]
[0163] The cured film of this invention is formed from this curable composition. This cured film has excellent solvent resistance. Such a cured film can be suitably used for applications requiring high surface hardness, for example, it can be suitab...
Embodiment
[0200] Hereinafter, based on an Example, this invention is demonstrated more concretely. The present invention is not limited by these Examples.
[0201] First, the measurement method and evaluation method of the physical properties will be described.
[0202] [ 1 Determination of H-NMR]
[0203] 1 H-NMR (Nuclear Magnetic Resonance, NMR) was measured at 25° C. using a nuclear magnetic resonance apparatus (“JNM-ECS400” (trade name) (400 MHz) of JEOL Ltd.).
[0204] [Measurement of weight average molecular weight Mw]
[0205] The weight average molecular weight Mw is a polystyrene equivalent molecular weight measured using a gel permeation chromatography (GPC) apparatus (Showa Denko's "GPC-101" (trade name)). The measurement with the GPC device uses a combination of "GPC-KF-801", "GPC-KF-802", "GPC-KF-803" and "GPC-KF-804" (the above are trade names, Showa Denko Corporation) The resulting column was used as a GPC column, and tetrahydrofuran (tetrahydrofuran, THF) was used ...
Synthetic example 1
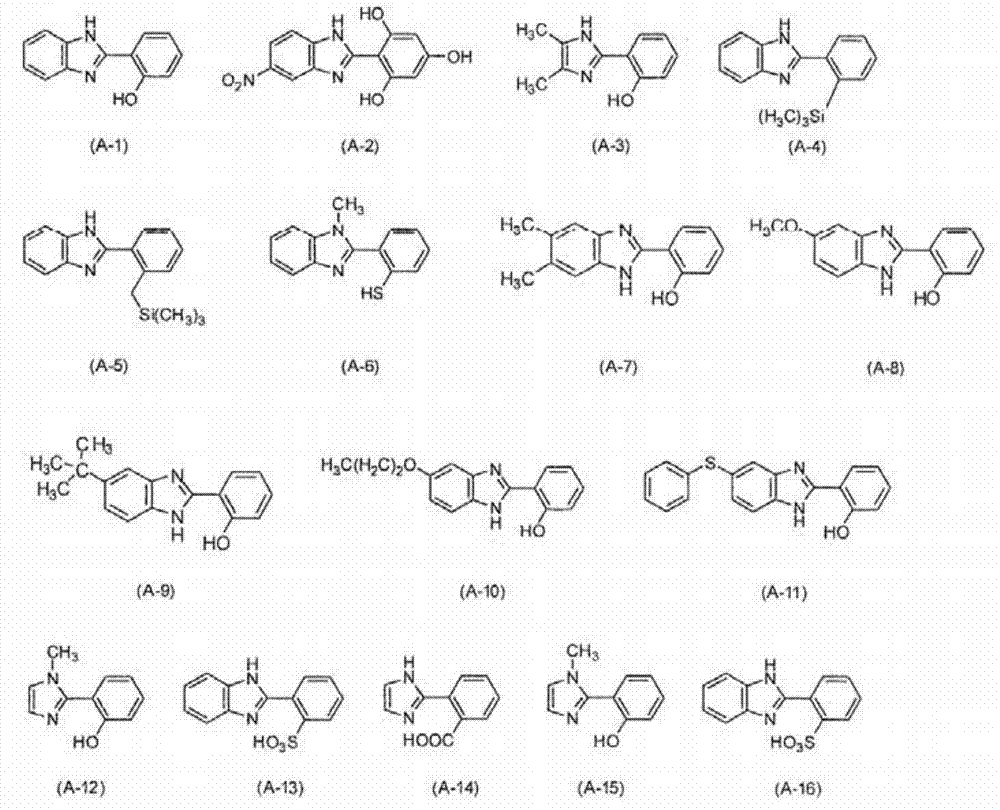
[0208] A compound represented by the following formula (A-1) (hereinafter also referred to as "compound (A-1)") was synthesized according to the following synthesis scheme.
[0209] [chemical 5]
[0210]
[0211] Add o-phenylenediamine (Sigma-Aldrich (Sigma-Aldrich) company) 3.24g (30.0mmol), salicylaldehyde (Sigma-Aldrich company) 3.67g (30.0mmol), sodium metabisulfite (Sigma-Aldrich company) in single-necked flask Derich Company) 5.70 g (30.0 mmol) and N, N-dimethylformamide (Sigma-Aldrich Company) 270 mL, heated to 80° C. in an oil bath, and reacted for 24 hours. After the reaction, the temperature was returned to room temperature, and the reaction solution was added to 300 mL of water at 0°C. The precipitate was collected by filtration, and purified by silica gel column chromatography to obtain compound (A-1).
[0212] Determination of compound (A-1) 1 H-NMR, the result is as described below.
[0213] 1 H-NMR (DMSO-d 6 );
[0214] δ7.00-7.04(2H, m), δ7.24-7.40(3H...
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
| pore size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com