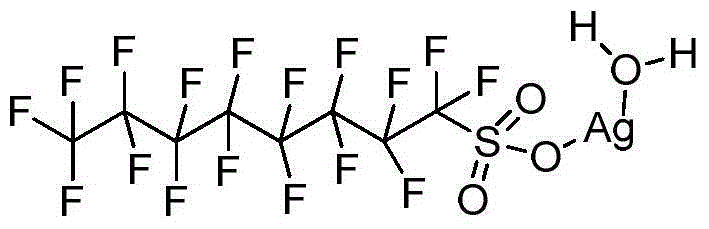
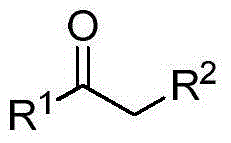
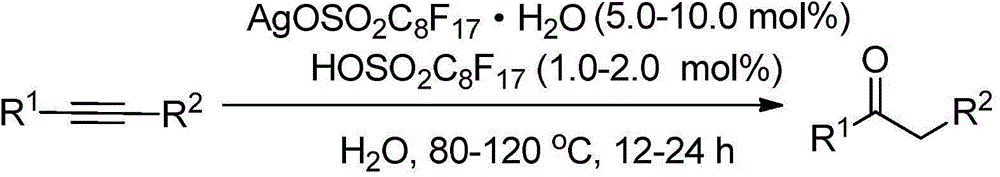Method for synthesizing ketone through catalyzing and hydrolyzing alkyne with silver perfluorooctanesulfonate
A technology for catalyzing alkynes with silver perfluorosulfonate and silver salts, which is applied in the field of catalysis to achieve good selectivity, good yield, and simple separation of products
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example 1
[0019] Add 0.05 mmol perfluorooctane sulfonate silver hydrate (AgOSO 2 C 8 f 17 ·H 2 O), 0.01mmol perfluorooctane sulfonic acid (HOSO 2 C 8 f 17 ) and 3.0mLH 2 O, 1mmol phenylacetylene (R 1 = Ph, R 2 =H) Then the flask was placed in an oil bath reactor with magnetic stirring, and the reaction was carried out at 100° C. for 12 hours. After the reaction was completed, 5ml of n-hexane was added for extraction and separation, the catalyst water in the lower layer was repeatedly catalytically utilized, and the upper layer liquid was subjected to column chromatography to obtain the product acetophenone. The result of the reaction is: the yield of acetophenone is 83%. After the catalyst system can be reused 5 times, its catalytic performance is slightly reduced, and the yield is 70%.
preparation example 2
[0021] Add 0.05 mmol perfluorooctane sulfonate silver hydrate (AgOSO 2 C 8 f 17 ·H 2 O), 0.02mmol perfluorooctane sulfonic acid (HOSO 2 C 8 f 17 ) and 3.0mLH 2 O, 1mmol phenylacetylene (R 1 = Ph, R 2 =H) Then the flask was placed in an oil bath reactor with magnetic stirring, and the reaction was carried out at 100° C. for 12 hours. After the reaction was completed, 5ml of n-hexane was added for extraction and separation, the catalyst water in the lower layer was repeatedly catalytically utilized, and the upper layer liquid was subjected to column chromatography to obtain the product acetophenone. The reaction results are: the yield of acetophenone is 94%, and the selectivity is greater than 95%. After the catalyst system can be reused five times, its catalytic performance is slightly reduced, and the yield is 83%.
preparation example 3
[0023] Add 0.1 mmol perfluorooctane sulfonate silver hydrate (AgOSO 2 C 8 f 17 ·H 2 O), 0.02mmol perfluorooctane sulfonic acid (HOSO 2 C 8 f 17 ) and 3.0mLH 2 O, 1mmol phenylacetylene (R 1 = Ph, R2 =H) Then the flask was placed in an oil bath reactor with magnetic stirring, and the reaction was carried out at 100° C. for 12 hours. After the reaction was completed, 5ml of n-hexane was added for extraction and separation, the catalyst water in the lower layer was repeatedly catalytically utilized, and the upper layer liquid was subjected to column chromatography to obtain the product acetophenone. The result of the reaction is: the yield of acetophenone is 94%. After the catalyst system can be reused 5 times, its catalytic performance is slightly reduced, and the yield is 86%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com