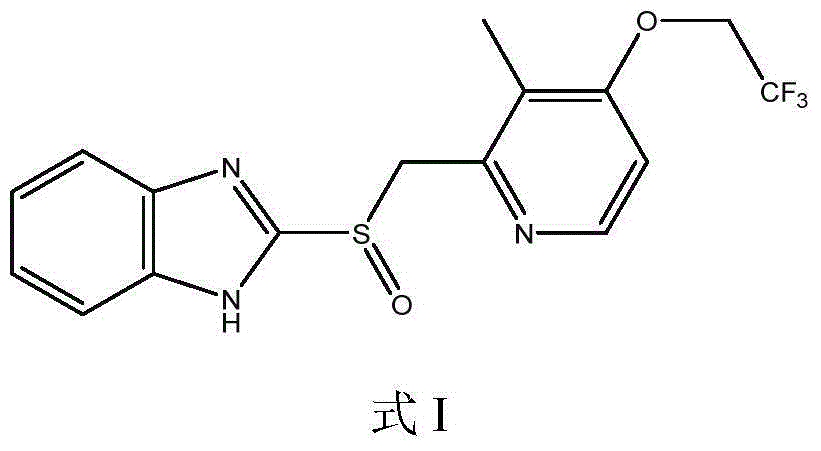
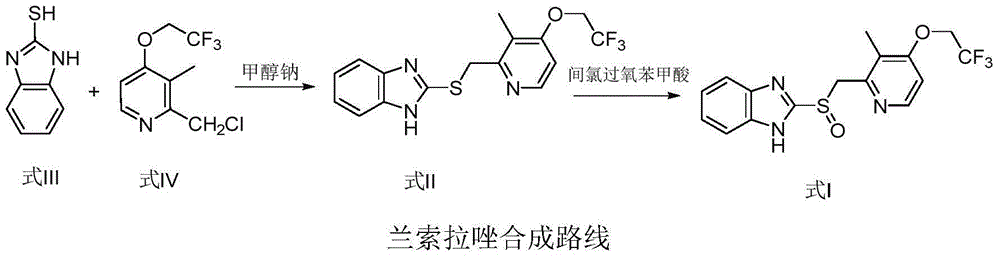
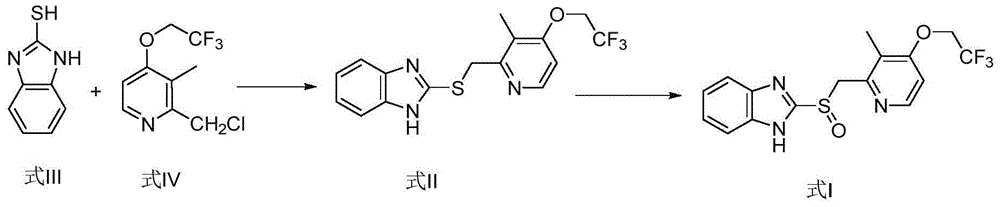
Lansoprazole preparation method
A technology for lansoprazole and compounds, which is applied in the field of "one-pot method" for preparation of lansoprazole compounds, can solve the problems of unfavorable energy saving, consumption reduction and link protection, difficult removal of oxidant impurities, and difficult control of reaction conditions, etc. Short production cycle, low solvent toxicity, mild and controllable reactions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] Add 500ml of 95% ethanol to the reaction flask, add 120g of 2-chloromethyl-3-methyl-4-(2,2,2-trifluoroethoxy)pyridine hydrochloride under stirring, and control the temperature at 25-35°C Add dropwise the solution of 65.16g 2-mercaptobenzimidazole and 43.56g sodium hydroxide in 350ml95% ethanol, control temperature reaction 2-3h, TLC monitoring reaction is complete (developing agent: dichloromethane: ethyl acetate=9: 1) Add 10% hydrochloric acid dropwise to adjust the pH to 6-7, a large amount of solids are precipitated, stirred and cooled to room temperature and then left to stand for 30 minutes, the reaction liquid is removed, and the solids in the reaction bottle are sampled to detect that the HPLC content is 99.8%. Then add 1000ml of ethanol to the reaction bottle, start stirring to dissolve all the solids, control the temperature at 10-20°C, slowly add 180g of 10% hydrogen peroxide dropwise, and then add 500ml of purified water after the reaction, a large amount of s...
Embodiment 2
[0028] Add 600ml of 90% ethanol to the reaction flask, add 120g of 2-chloromethyl-3-methyl-4-(2,2,2-trifluoroethoxy)pyridine hydrochloride under stirring, and control the temperature at 25-35°C Add dropwise the solution of 65.16g 2-mercaptobenzimidazole and 43.56g sodium hydroxide in 350ml90% ethanol, control the temperature and react for 2-3h, TLC monitors that the reaction is complete (developing solvent: dichloromethane: ethyl acetate=9: 1), add dropwise 5% hydrochloric acid to adjust the pH to 6-7, a large amount of solids are precipitated, stirred and cooled to room temperature and then left to stand for 30 minutes, the reaction liquid is removed, and the solid in the reaction bottle is sampled to detect that the HPLC content is 99.8%. Then add 900ml of ethanol to the reaction bottle, start stirring to dissolve all the solids, control the temperature at 10-20°C, slowly add 180g of 10% hydrogen peroxide dropwise, and then add 900ml of purified water after the reaction, a la...
Embodiment 3
[0030] Add 600ml of 95% ethanol to the reaction flask, add 120g of 2-chloromethyl-3-methyl-4-(2,2,2-trifluoroethoxy)pyridine hydrochloride under stirring, and control the temperature at 25-35°C Add dropwise the solution of 65.16g 2-mercaptobenzimidazole and 43.56g sodium hydroxide in 350ml95% methanol, control the temperature and react for 2-3h, and TLC monitors that the reaction is complete (developing solvent: dichloromethane: ethyl acetate=9: 1), adding 5% sulfuric acid dropwise to adjust the pH to 6-7, a large amount of solids were precipitated, stirred and cooled to room temperature, and the reaction clear liquid was removed, and the solids in the reaction flask were sampled to detect that the HPLC content was 99.8%. Then add 1000ml of ethanol to the reaction bottle, start stirring to dissolve all the solids, control the temperature at 10-20°C, slowly add 180g of 10% hydrogen peroxide dropwise, and then add 800ml of purified water after the reaction, a large amount of soli...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com