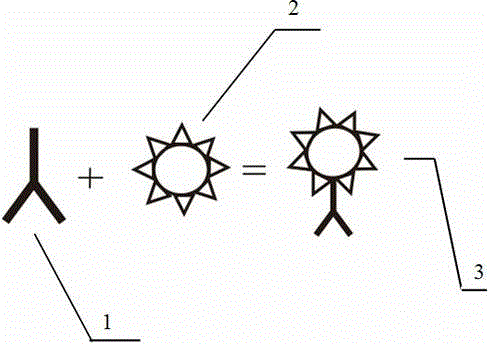
Preparation method of polystyrene fluorescent microsphere coupled with antibody
A technology of fluorescent microspheres and polystyrene, applied in fluorescence/phosphorescence, material excitation analysis, etc., can solve the problems of strict experimental conditions and complicated fluorescein labeling process, and achieve good dispersion, weakened agglomeration, and specific binding enhanced effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] Step 1: Use 0.01M MES buffer as the activation buffer, take 100 μL of fluorescent microspheres with carboxyl groups on the surface and add them to a 1.5 mL centrifuge tube, wash with MSE buffer for 3 times, discard the supernatant in a refrigerated centrifuge, and then centrifuge Add MES buffer to the tube, add freshly prepared 0.5g / mL EDC solution and 0.25g / mL sulfo-NHS solution at a volume ratio of 1:2, shake and mix with a vortex mixer, and rotate to activate the carboxyl groups on the surface of the microspheres at room temperature 10 Minutes, and then wash the fluorescent microspheres with MSE buffer 2 times.
[0034] Step 2: Use 0.02M BST buffer as the buffer in the coupling process. After washing, add 10 μg of monoclonal antibody, then add BST buffer, keep the concentration of fluorescent microspheres at 3 mg / ml, and rotate for 1 hour at room temperature, then The supernatant was separated and collected by a refrigerated centrifuge, and the coupling rate was ...
Embodiment 2
[0038]Step 1: Use 0.01M MES buffer as the activation buffer, add 100 μL of fluorescent microspheres with carboxyl groups on the surface into a 1.5 mL centrifuge tube, wash with MSE buffer for 3 times, then use a refrigerated centrifuge to discard the supernatant. After washing 3 times, add MES buffer to the centrifuge tube, add freshly prepared 0.5g / mL EDC solution and 0.25g / mL sulfo-NHS solution at a volume ratio of 1:2, shake and mix with a vortex mixer, and rotate at room temperature Activate the carboxyl groups on the surface of the microspheres for 10 minutes, then wash the microspheres twice with MSE buffer.
[0039] In step 2, use 0.02M BST buffer as the buffer for the coupling process. After washing, add 10 μg of monoclonal antibody, and then add BST buffer, rotate and react at room temperature for 2 hours, then separate and collect the supernatant with a refrigerated centrifuge, and detect that the coupling rate is 85%.
[0040] Step 3, add BST buffer and 5.0 μL glyc...
Embodiment 3
[0043] Step 1: Use 0.01M MES buffer as the activation buffer, add 100 μL of fluorescent microspheres with carboxyl groups on the surface into a 1.5 mL centrifuge tube, wash with MSE buffer for 3 times, then use a refrigerated centrifuge to discard the supernatant. After washing 3 times, add MES buffer to the centrifuge tube, add freshly prepared 0.5g / mL EDC solution and 0.25g / mL sulfo-NHS solution at a volume ratio of 1:2, shake and mix with a vortex mixer, and rotate at room temperature Activate the carboxyl groups on the surface of the microspheres for 10 minutes, then wash the microspheres twice with MSE buffer.
[0044] In step 2, use 0.02M BST buffer as the buffer for the coupling process. After washing, add 10 μg of monoclonal antibody, and then add BST buffer, rotate at room temperature for 2 hours, then separate and collect the supernatant with a refrigerated centrifuge, and detect that the coupling rate is 93%.
[0045] Step 3, add BST buffer and 7.5 μL glycine t...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
| coupling rate | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com