Preparation method of anhydrous magnesium chloride
A technology of anhydrous magnesium chloride and magnesium chloride, which is applied in the direction of magnesium chloride and magnesium halide, can solve the problems of difficulty in dehydration of low-hydrate magnesium chloride, difficulty in improving the purity of the final product, and easy corrosion of equipment, so as to achieve easy industrialization, low price, and low energy consumption. consumption effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
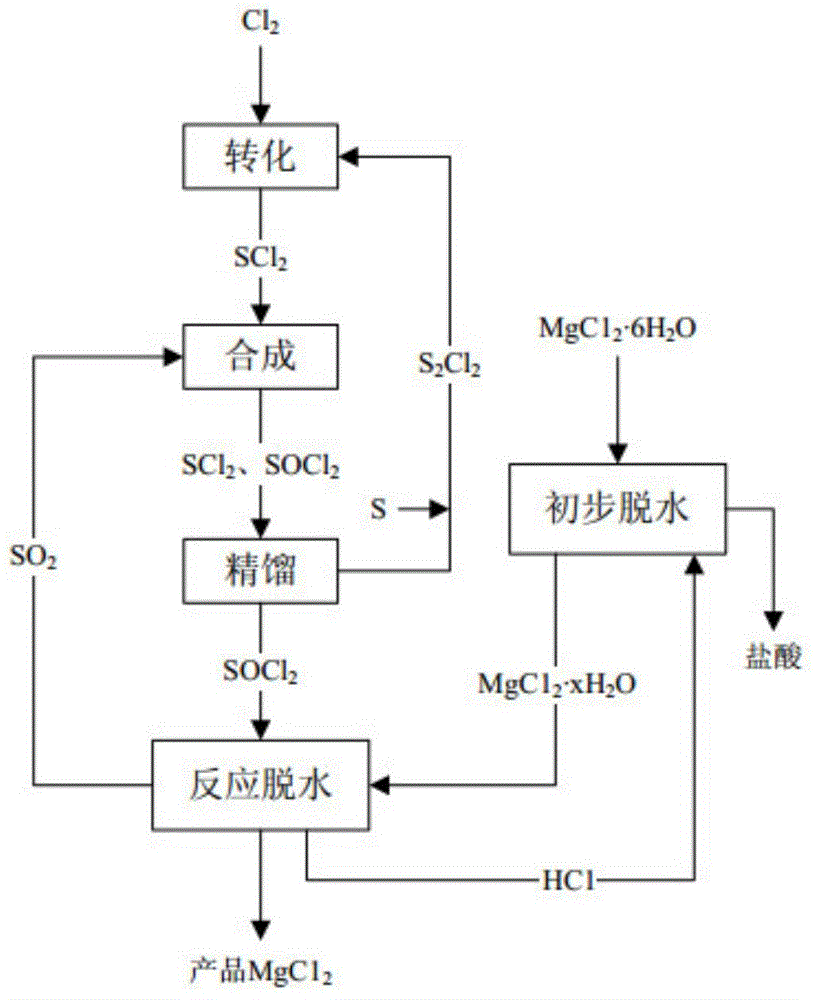
[0035] seefigure 1 , figure 1 The process flow chart of the preparation method of the anhydrous magnesium chloride that the embodiment of the present invention provides, from figure 1 Visible in, the preparation method of anhydrous magnesium chloride comprises the steps:
[0036] 1) The magnesium chloride high hydrate is dehydrated by physical temperature rise to obtain the magnesium chloride low hydrate MgCl 2 ·xH 2 O;
[0037] Specifically, under the protection of HCl gas flow, the magnesium chloride high hydrate is slowly heated in a fluidized bed, and part of the water is initially removed to form a low hydrate MgCl 2 ·xH 2 O;
[0038] Preferably, magnesium chloride high hydrate is MgCl 2 ·6H 2 O, it can be understood that the magnesium chloride high hydrate of the present invention is MgCl 2 ·6H 2 O;
[0039] Wherein, in the obtained low hydrate MgCl2 xH2O, the numerical range of x is 0.5<x<2.5;
[0040] Wherein, the temperature of physical heating and dehydrat...
Embodiment 1
[0056] Under the protection of HCl gas flow, the magnesium chloride high hydrate is slowly heated to 100°C in a fluidized bed, and part of the water is initially removed to form a low hydrate MgCl 2 ·xH 2 O; pass Cl at 55°C 2 with S from subsequent distillation section 2 Cl 2 Mix and react the S 2 Cl 2 into SCl 2 ; Under the condition of 150 ℃, the SCl obtained by the above reaction 2 SO with follow-up segment returns 2 Reaction to synthesize SOCl 2 ; Under the condition of 50°C, the reaction product obtained in the above steps is passed into a rectification tower for rectification, and SOCl is obtained at the bottom of the tower 2 ; Under the condition of 60°C, the obtained SOCl 2 Low water compound MgCl obtained through physical biological dehydration 2 ·xH 2 In O, a chemical reaction occurs to dehydrate to obtain the product high-purity anhydrous MgCl 2 .
Embodiment 2
[0058] Under the protection of HCl gas flow, the magnesium chloride high hydrate is slowly heated to 200°C in a fluidized bed, and part of the water is initially removed to form a low hydrate MgCl 2 ·xH 2 O; pass Cl at 65°C 2 with S from subsequent distillation section 2 Cl 2 Mix and react the S 2 Cl 2 into SCl 2 ; Under the condition of 200 ℃, the SCl obtained by the above reaction 2 SO with follow-up segment returns 2 Reaction to synthesize SOCl 2 ; Under the condition of 100 ° C, the reaction product obtained in the above steps is passed into a rectification tower for rectification, and SOCl is obtained at the bottom of the tower 2 ; Under the condition of 80 ℃, the obtained SOCl 2 Low water compound MgCl obtained through physical biological dehydration 2 ·xH 2 In O, a chemical reaction occurs to dehydrate to obtain the product high-purity anhydrous MgCl 2 .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com