Novel growth hormone releasing hormone analog peptides and application thereof in preparing medicines for treating infertility
A growth hormone and hormone-releasing technology, applied in the field of medicine and biology, can solve the problems of low activity and short half-life
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
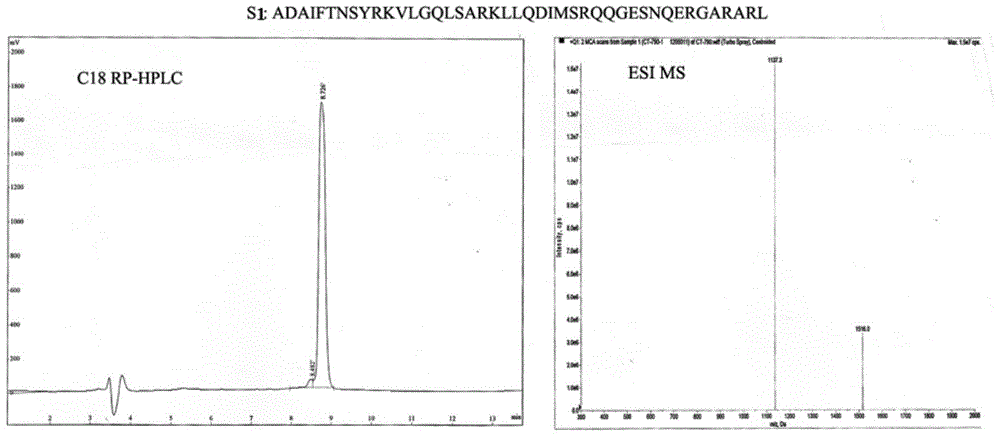
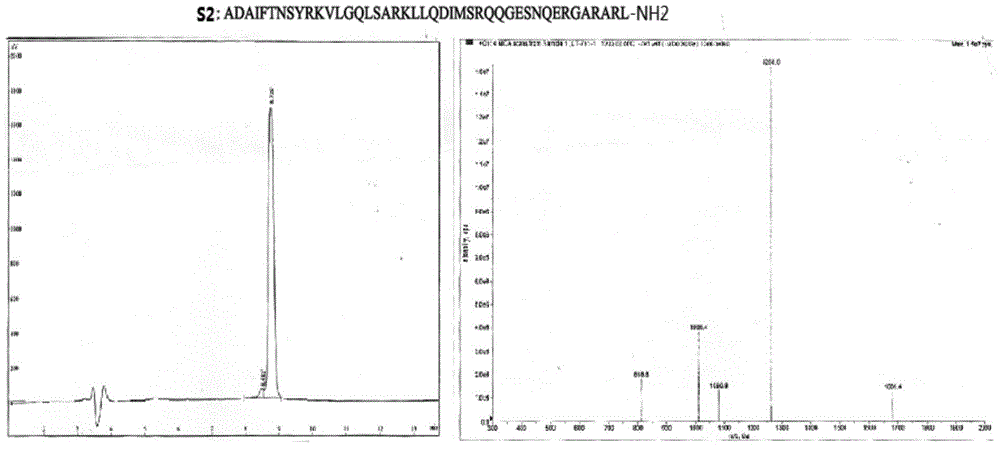
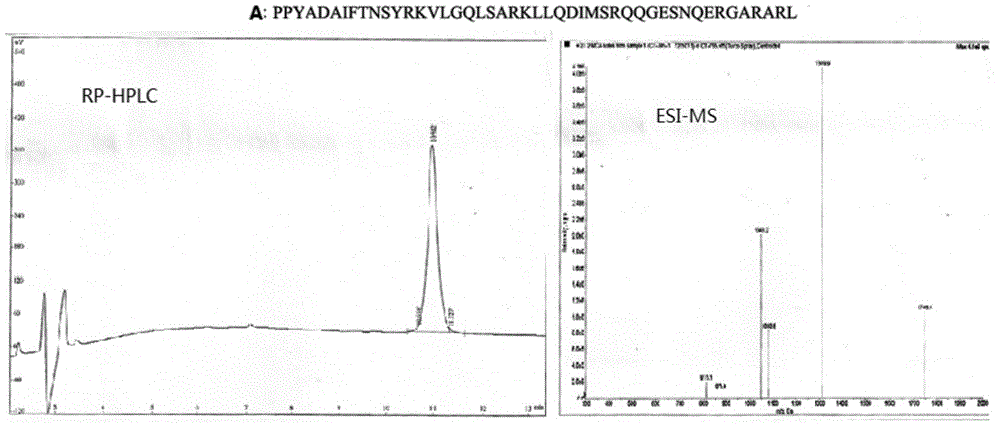
[0046] Example 1: Synthesis of novel growth hormone releasing hormone analogues:
[0047] 1. Monomer peptide synthesis process: manual chemical solid-phase peptide synthesis (SYMPHONY 12-channel peptide synthesizer, software version.201, Protein Technologies Inc.), operation steps:
[0048] 1. Resin swelling: put Dawang resin (wang resin, purchased from Tianjin Nankai Synthetic Technology Co., Ltd.) into a reaction pot, add dichloromethane (DCM, Dikma Technologies Inc.) 15ml / g in Dawang resin, shake 30min.
[0049] 2. Connect the first amino acid: Remove the solvent through sand core suction filtration, add 3 mmol of the first Fmoc-AA amino acid at the C-terminal (all Fmoc-amino acids are provided by Suzhou Tianma Pharmaceutical Group Fine Chemicals Co., Ltd.), and then Add 4-dimethylaminopyridine (DMAP) and N,N'-dicyclohexylcarbodiimide (DCC) in 10 millimolar amounts, and finally dimethylformamide (DMF) (purchased from Dikma Technologies Inc.) Dissolve and shake for 30min. ...
Embodiment 2
[0072] Example 2: Hormone releasing activity in vitro:
[0073] 1. In vitro pituitary gland stimulation method: S-D female mice (about 8 weeks old, weighing 200±20 g) were purchased from the Experimental Animal Center of Guangzhou University of Traditional Chinese Medicine. All experimental animals were kept and used in accordance with institutional guidelines. During feeding, the rats were reared alternately between light and dark at 12:12 hours, and the feeding temperature was 26±1°C. Rats died suddenly by dislocation, and all pituitary glands were removed within 30 minutes. After washing with sterilized lactated Ringer's buffer (LRB), the pituitary gland was immediately placed in a 1×10 cm glass tube with 1 ml of LRB, and kept at 37°C. All glass tubes are kept warm for 5h (P 1 ,P 2 ,I 3 ,I 4 and I 5 , representing different periods of 5-hour incubation, and each period represents 1 hour), while shaking gently every 5 minutes, after every 1 hour, gently suck out the b...
Embodiment 3
[0080] Embodiment 3: Rat pituitary growth hormone release inhibition analysis:
[0081] According to the pituitary GH release activity test (P 1 , P 2 , I 3 , I 4 , I 5 ), can determine growth hormone release inhibition method, in I 3 -I 5 During the incubation, 0.482 or 1.927 μM growth hormone inhibitory hormone (GHIH) was added to tubes containing 1.927 μM GHRH dimer, respectively. No peptide P 2 The incubation was used as a blank and the S2 peptide was used as a standard control. Rat pituitary growth hormone levels were determined by rat growth hormone ELISA kit.
[0082] According to the 5-h pituitary culture assay, at I 3 -I 5 During the incubation, when 1.927 μM GHRH peptide appeared, the addition of 0.482 or 1.927 μM GHIH peptide showed a significant inhibitory effect, and the inhibitory effect was proportional to the GHIH peptide dose and incubation time ( Figure 14 ). in I 3 -I 5 Among them, the inhibition of 1.927 μM GHIH was significantly more than th...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com