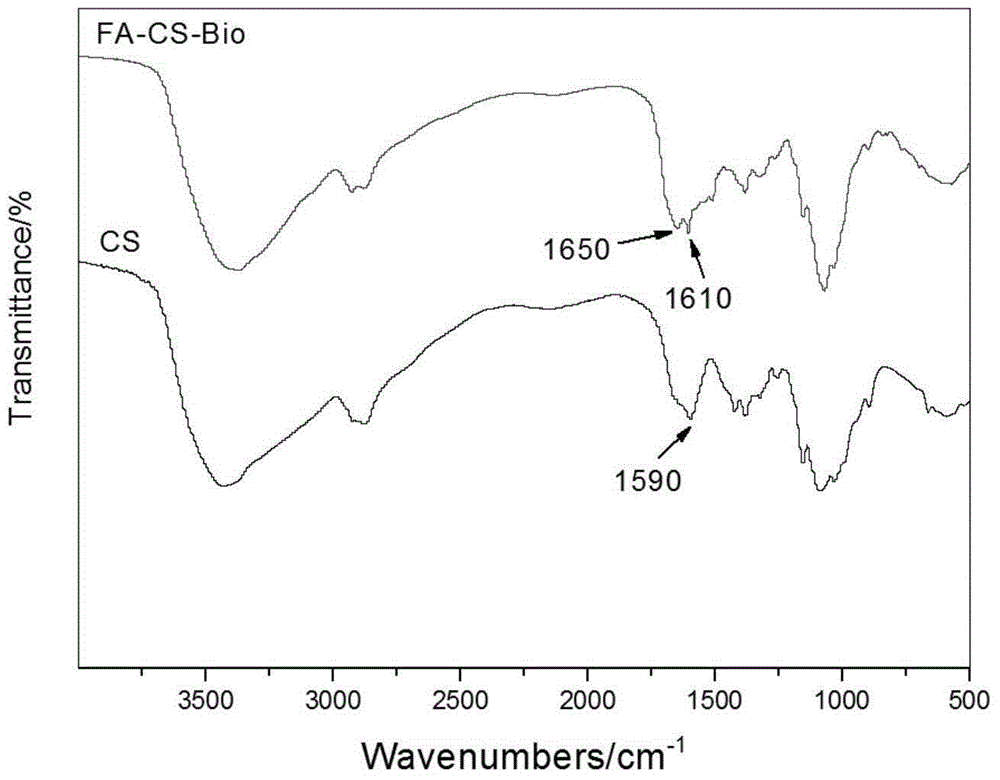
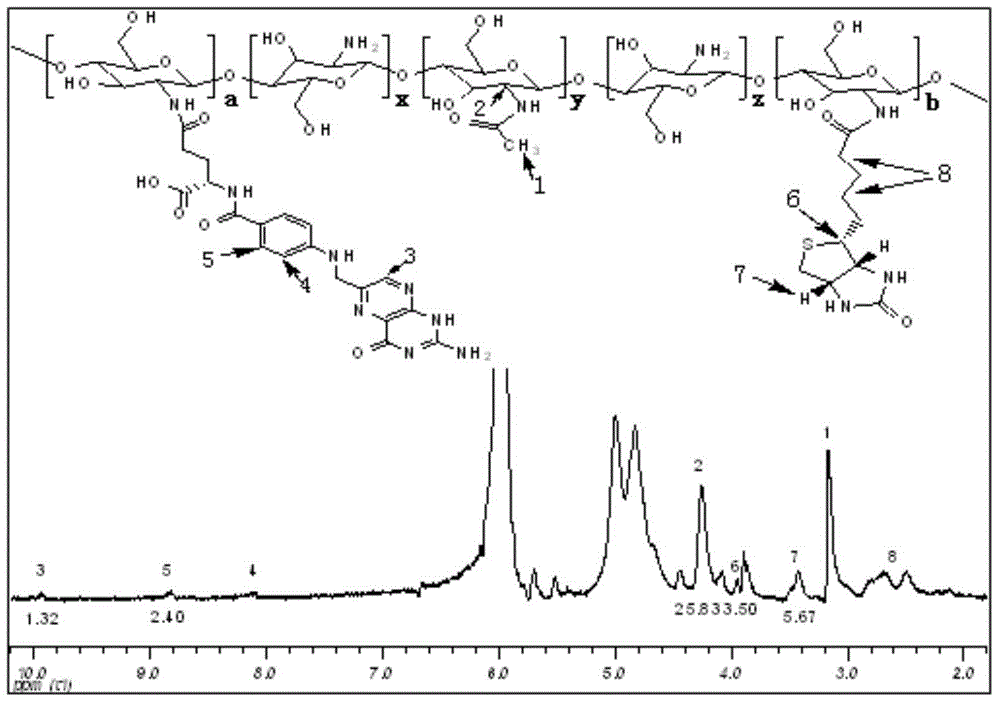
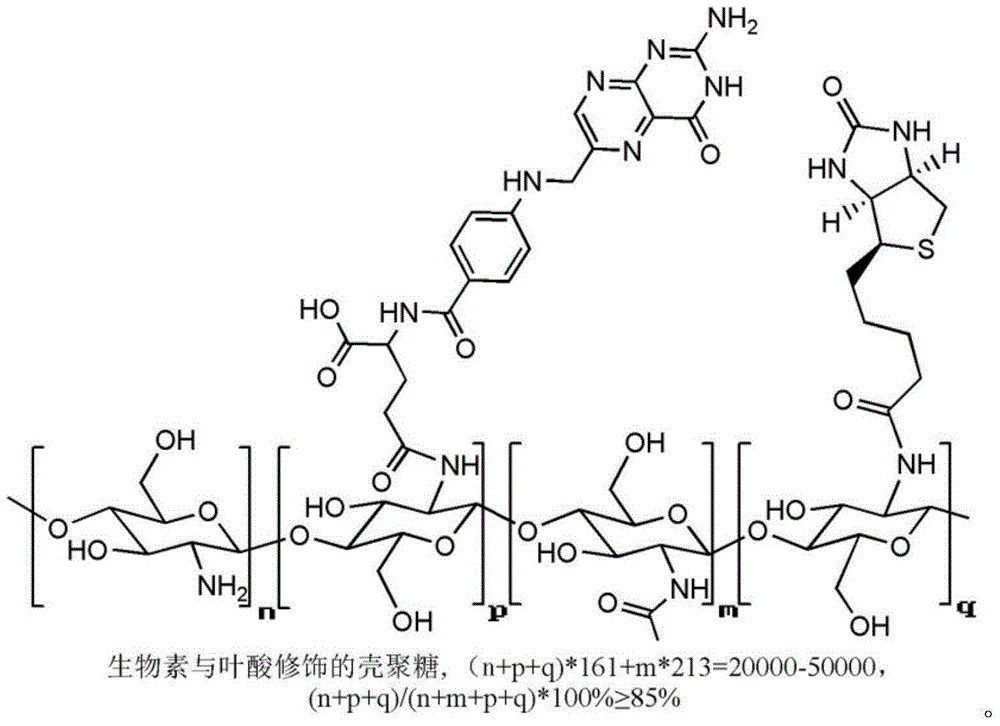
Folacin/biotin modified chitosan material and preparation method thereof
A chitosan and biotin technology, applied in the field of folic acid/biotin modified chitosan material and its preparation, can solve problems such as unreported, and achieve strong specific recognition, shortened reaction time, and targeted reliable results
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] Synthesis of folate / biotin modified chitosan material (FA-CS-Bio):
[0032] Weigh 0.1g chitosan (M w =50000, D.D=85%), fully dissolved in 20mL 1% (v / v) HAc / H 2 In O, add the diluted NaOH solution dropwise, adjust the pH to 4.7, and obtain the chitosan solution;
[0033] Weigh 2.7g, 1.01g, and 2.11g of DCC, NHS, and biotin respectively, add them into a three-necked flask and mix them, and then react at 60°C for 24 hours. Then recrystallize the precipitate with isopropanol, collect the crystals by filtration and dissolve the crystals with DMF, add dropwise to anhydrous ether after filtration, refrigerate overnight, remove the supernatant, and dry under vacuum at room temperature to obtain succinimidyl biotin ester, spare;
[0034] Weigh 26.5mg of folic acid and 115.0mg of EDC.HCl and dissolve in 1mL of anhydrous DMSO at the same time, stir and activate at 40°C for 1 hour in the dark to obtain folic acid active ester solution;
[0035] Weigh 20.5mg of succinimidyl biot...
Embodiment 2
[0040] Synthesis of folate / biotin modified chitosan material (FA-CS-Bio):
[0041] Weigh 0.1g chitosan (M w=20000, D.D=91%), fully dissolved in 10mL 1% (v / v) HAc / H 2 In O, add the diluted NaOH solution dropwise, adjust the pH to 6, and obtain the chitosan solution;
[0042] Weigh 2.16g, 1.01g, and 2.11g of DCC, NHS, and biotin respectively, add them into a three-necked flask and mix them, and then react at 50°C for 16 hours. After filtering, diethyl ether is added dropwise to the filtrate in an ice bath to precipitate precipitation. Then recrystallize the precipitate with isopropanol, collect the crystals by filtration and dissolve the crystals with DMF, add dropwise to anhydrous ether after filtration, refrigerate overnight, remove the supernatant, and dry under vacuum at room temperature to obtain succinimidyl biotin ester, spare;
[0043] Weigh 44.1mg of folic acid and 95.9mg of EDC.HCl and dissolve in 1mL of anhydrous DMSO at the same time, stir and activate in the dark...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com