A method for preparing 7α, 15α-dihydroxydhydroepiandrosterone by using Trichospora flaxensis
A technology of Trichosporium flaxensis and dehydroepiandrosterone, applied in the field of hydroxylation, can solve problems such as poor effect, difficult product separation, and expansion of production scale
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
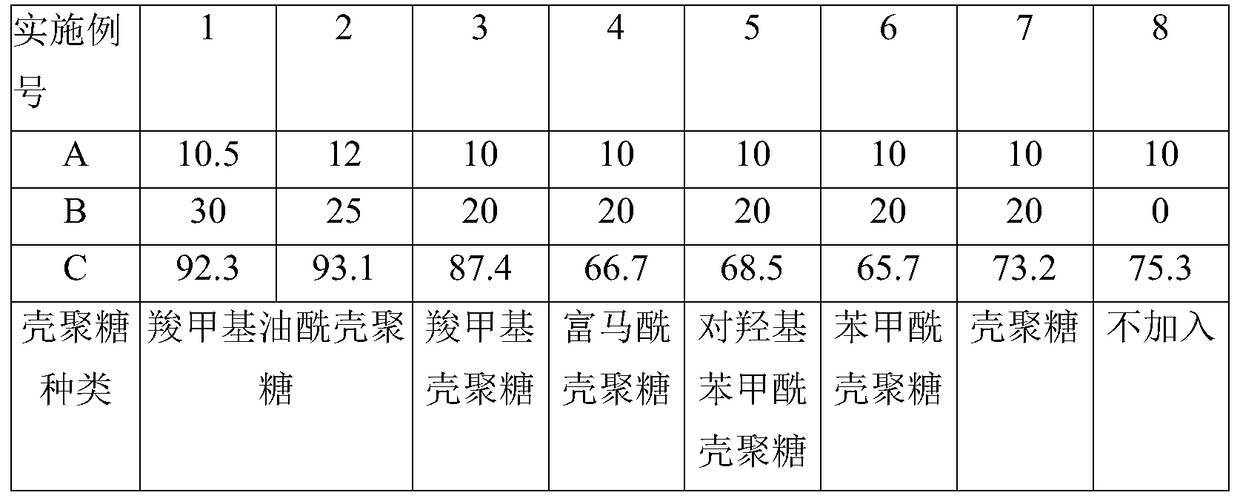
[0022] Below in conjunction with the examples, the specific implementation of the present invention will be further described in detail. The following examples are used to illustrate the present invention, but are not intended to limit the scope of the present invention.
[0023] Strain: Colletotrichum lini ST-1, preservation number CGMCC No.6051
[0024] Substrate raw material: dehydroepiandrosterone (DHEA), content 98%,
[0025] Fumaryl chitosan, p-hydroxybenzoyl chitosan, and benzoyl chitosan are all according to Reference 1—Study on the chemical modification of chitosan and the antibacterial activity of its derivatives (Feng Yongwei, Jiangnan University Doctoral dissertation, June 2011), prepared by the disclosed method, wherein the degree of substitution of carboxymethyl oleoyl chitosan is 0.45-0.48, the degree of substitution of benzoyl chitosan is 1.2-1.3, and the degree of substitution of p-hydroxybenzoyl chitosan is 0.45-0.48. The degree of substitution of acyl chit...
PUM
| Property | Measurement | Unit |
|---|---|---|
| degree of substitution | aaaaa | aaaaa |
| degree of substitution | aaaaa | aaaaa |
| degree of substitution | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com